Abstract
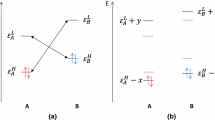
The recently proposed similarity index was applied to the quantitative justification of the empirical Hammond postulate for a series of selected pericyclic reactions. In keeping with the expectations of the postulate the transition states were shown to be reactant-like for exothermic and product-like for endothermic reactions.
Similar content being viewed by others
References
D.H. Rouvray, J. Chem. Inf. Comp. Sci. 32 (1992) 580.
M.A. Johnson and G.M. Maggiora (eds.),Concepts and Applications of Molecular Similarity (Wiley, New York, 1990).
R.I. Zalewski, T.M. Krygowski and J. Shorter (eds.),Similarity Models in Organic Chemistry, Biochemistry and related Fields (Elsevier, Amsterdam, 1991).
R. Carbô (ed.),Molecular Similarity and Reactivity. From Quantum Chemical to Phenomenological Approaches (Kluwer, Dordrecht, 1995).
K.D. Sen (ed.),Topics in Current Chem., Vols. 173,174 (Springer, Berlin, 1995).
W. Huckel, Theoretische Grundlagen der Organischen Chemie (Akad. Verlaggesselsch., Leipzig, 1934).
F.O. Rice and E. Teller, J. Chem. Phys. 6 (1938) 489.
J. Hine, J. Am. Chem. Soc. 88 (1966) 5525.
O.S. Tee and K. Yates, J. Am. Chem. Soc. 94 (1972) 3074.
S. Ehrenson, J. Am. Chem. Soc. 96 (1974) 3778, 3784.
M.L. Sinnot, Adv. Phys. Org. Chem. 24 (1988) 113.
A. Igawa and H. Fukutome, Chem. Phys. Lett. 133 (1987) 1180.
C. Jochum, J. Gasteiger, I. Ugi and J. Dugundji, Z. Naturforsch. (b) 37 (1982) 1205.
I. Ugi, M. Wochner, E. Fontain, J. Bauer, B. Gruber and R. Karl, in:Concepts and Applications of Molecular Similarity, eds. M.A. Johnson and G.M. Maggiora (Wiley, New York, 1990) chap. 9.
C. Jochum, J. Gasteiger and I. Ugi, Angew. Chem. Int. Ed. 8 (1980) 495.
O.E. Polansky and O.E.G. Derflinger, Int. J. Quant. Chem. 1 (1967) 379.
R. Carbó, L. Leyda and M. Arnau, Int. J. Quant. Chem. 17 (1980) 1185.
D.L. Cooper and N.L. Allan, J. Comp. Aided Mol. Design 3 (1989) 253.
E.E. Hodgkin and W.G. Richards, J. Chem. Soc. Chem. Commun. (1986)1342.
J. Cioslowski and E.D. Fleischmann, J. Am. Chem. Soc. 113 (1991) 64.
R. Ponce, Collect. Czech. Chem. Commun. 52 (1987) 555.
Proc. Beilstein Workshop on the Similarity in Organic Chemistry, J. Chem. Inf. Comp. Sci. 32 (Special Issue) (1992).
G.S. Hammond, J. Am. Chem. Soc. 77 (1955) 334.
M. Sola, J. Mestres, R. Carbó and M. Duran, J. Am. Chem. Soc. 116 (1994) 5909.
J. Cioslowski, J. Sauer, J. Hetzenegger, T. Katcher and T. Hierstetter, J. Am. Chem. Soc. 115 (1993)1353.
G.A. Arteca and P.G. Mezey, Int. J. Quant. Chem. Quant. Chem. Symp. 24 (1990) 1.
G.A. Arteca and P.G. Mezey, J. Phys. Chem. 93 (1989) 4746.
G.A. Arteca and P.G. Mezey, J. Comput. Chem. 9 (1988) 728.
R. Ponec and M. Strnad, J. Chem. Inf. Comp. Sci. 32 (1992) 693.
R. Ponec, Z. Phys. Chem. (Leipzig) 268 (1987) 1180.
R. Ponec, Z. Phys. Chem. (Leipzig) 270 (1989) 365.
R. Ponec and M. Strnad, J. Math. Chem. 8 (1990) 108.
R. Ponec and M. Strnad, J. Phys. Org. Chem. 4 (1991) 701.
R. Ponec and M. Strnad, J. Phys. Org. Chem. 5 (1992) 764.
R. Ponec, Topics in Curr. Chem. 174 (1995) 1.
L. Salem, in:Electrons in Chemical Reactions (Wiley, New York, 1982) chap. 2.
R. Ponce, Collect. Czech. Chem. Commun. 49 (1984) 455.
R. Ponce and M. Strnad, Croat. Chem. Acta 66 (1993) 123.
C. Trindle, J. Am. Chem. Soc. 92 (1971) 3251.
J.M. Bofill, J. Gomez and S. Olivella, J. Mol. Struct. (Theochem) 163 (1988) 285.
M.J.S. Dewar, E.G. Zoebisch, E.F. Healy and J.P. Stewart, J. Am. Chem. Soc. 107 (1985) 3902.
J.P. Stewart, J. Comp. Aided Mol. Des. 4 (1990) 1.
D.C. Spellmayer and K.N. Houk, J. Am. Chem. Soc. 110 (1988) 3412.
M.J.S. Dewar, S. Olivella and J.P. Stewart, J. Am. Chem. Soc. 108 (1986) 5771.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ponec, R., Yuzhakov, G. & Pecka, J. Similarity approach to chemical reactivity. Theoretical justification of the Hammond postulate. J Math Chem 19, 265–270 (1996). https://doi.org/10.1007/BF01166718
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01166718