Summary
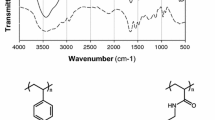
Dowex 50 W resin in the form of an ethylamine-Cu11 complex ion was used as potentially active catalyst for the decomposition of H2O2 in aqueous medium. The stoichiometry of the amine-Cu11 complex on the resin, determined experimentally, was found to have the total [Cu2+]: [ethylamine]=1∶4 concentration ratio. The kinetics of the decomposition was studied and the calculated rate constant (per g of dry resin) was found to decrease with increase the degree of resin crosslinking. The active species, formed as an intermediate at the beginning of the reaction, had an inhibiting effect on the reaction rate. The brown peroxo-copper complex formed as a result of H2O2 decomposition, was found to contain the catalytic active species. The order of the reaction increased with decreasing initial H2O2 concentration, a sign of a step-wise mechanism. A quantitative treatment of the decomposition of H2O2 was provided in terms of activation parameters.
Similar content being viewed by others
References
F. Helfferich,Ion Exchange, McGraw-Hill Book Co., Inc., New York, 1962, Ch. 5.
K. Hayakawa and S. Nakamura,Chem. Soc. Jpn., Bull.,47, 1162 (1974).
M. Y. El-Sheikh, F. M. Ashmawy, I. A. Salem and A. B. Zaki,Z. Phys. Chemie, Leipzig,268, 595 (1987).
T. Kaden and H. Sigel,Helvetica Chim. Acta.,51, 947 (1968).
H. Sigel, C. Flierl and R. Griesser,J. Am. Chem. Soc.,91, 1061 (1969).
G. E. Boyed and B. A. Soldano,J. Am. Chem. Soc.,75, 6091 (1954).
J. R. Clifton and J. T. Yoke,Inorg. Chem.,7, 39 (1968).
N. Hawkings, K. D. Horton and K. W. Snelling. Report (1980) AEEW-R-1390, from INIS Atomindex12 (1981), Abstr. No. 605735,cf.95, 20899b (1981).
C. W. Davies and G. G. Thomas,J. Chem. Soc., 1607 (1952).
G. Pannetier and P. Souchay,Chemical Kinetics, Elsevier publishing Co. Ltd., New York, 1967, Ch. 4.
J. H. Espenson,Chemical Kinetics and Reaction Mechanism, McGraw-Hill Book Co. Inc., New York, 1981, p. 93.
A. B. Zaki, G. B. El-Hefnawy, A. Habib and S. E. Morsi,Z. Phys. Chem., Leipzig,263, 161 (1982).
F. Helfferich,Ion Exchange, McGraw-Hill Book Co. Inc., New York, 1962, Ch. 11.
V. S. Sharma and J. Schubert,J. Am. Chem. Soc.,91, 6291 (1969).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
El-Sheikh, M.Y., Habib, AF.M., Ashmawy, F.M. et al. Ion Exchangers as catalysts I. Catalytic decomposition of hydrogen peroxide in presence of Dowex 50 W resin in the form of ethylamine-copper(II) complex ion in aqueous medium. Transition Met Chem 13, 96–100 (1988). https://doi.org/10.1007/BF01087796
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01087796