Abstract
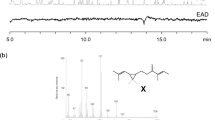
6-Acetoxy-5-hexadecanolide (Ia) in the oviposition attractant pheromone released from egg apical droplets of the mosquitoCulex pipiens fatigans Wied. is shown to be the (−)-(5R,6S)- enantiomer. Identification was by chromatography of the 6-trifluoroacetoxy derivatives of the natural pheromone and of the synthetic (−)-(5R,6S)- (Ib) and (+)-(5S,6R)- (IIb) enantiomers on a capillary column having a chiral stationary phase comprising a derivative of (1S,3S)-chrysanthemic acid. The synthetic (−)-(5R,6S)- enantiomer (Ia) attracted oviposition of four fold more mosquito egg rafts than the control (P < 0.01) whereas for the (5S,6R)- enantiomer (IIa) there was no statistically significant oviposition attraction.
Similar content being viewed by others
References
Bruno, D.W., andLaurence, B.R. 1979. The influence of the apical droplet ofCulex egg rafts on oviposition ofCulex pipiens fatigans (Diptera: Culicidae).J. Med. Entomol. 16:300–305.
König, W.A. 1982. Separation of enantiomers by capillary gas chromatography with chiral stationary phases.J. High-Resolution Chromatogr. Column Chromatogr. 5:588–595.
König, W.A., Francke, W., andBenecke, I. 1982. Gas Chromatographic enantiomer separation of chiral alcohols.J. Chromatogr. 239:227–231.
Laurence, B.R., andPickett, J.A. 1982.erythro-6-Acetxoy-5-hexadecanolide, the major component of a mosquito oviposition attractant pheromone.J. Chem. Soc., Chem. Commun. 59–60.
Laurence, B.R., andPickett, J.A. 1985. An oviposition attractant-pheromone inCulex pipiens fatigans Wisch. (Diptera: Culicidae).Bull. Ent. Res. (in press).
Mori, K., andOtsuka, T. 1983. Synthesis of both the enantiomers oferythro-6-acetoxy-5-hexad- ecanolide, the major component of a mosquito oviposition attractant pheromone.Tetrahedron 39:3267–3269.
Oi, N., Kitahara, H., andDoi, T. 1983. Gas Chromatographic separation of chrysanthemic acid ester enantiomers on a novel chiral stationary phase.J. Chromatogr. 254:282–284.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Laurence, B.R., Mori, K., Otsuka, T. et al. Absolute configuration of mosquito oviposition attractant pheromone, 6-acetoxy-5-hexadecanolide. J Chem Ecol 11, 643–648 (1985). https://doi.org/10.1007/BF00988573
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00988573