Abstract
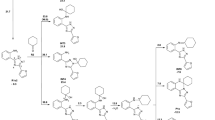
The structures of previously obtained nucleosides of 5-substituted 4-chloro-1, 2,3-triazoles were refined by means of high-resolution mass spectrometry and 13C NMR spectroscopy. It is shown that fusion of 5-substituted 4-chloro,1,2,3-triazoles with tetra-0-acylribofuranoses in the presence of di(p-nitrophenyl) phosphate leads to the formation of 2-nucleosides of the corresponding triazoles. The signals of the carbon atoms in the 13C NMR spectra of the 4,5-di-substituted triazoles and their nucleosides were assigned.
Similar content being viewed by others
Literature Cited
I. D. Shingarova, I. V. Yartseva, M. P. Nemeryuk, A. L. Sedov, G. A. Osipov, Yu. Yu. Volodin, and M. N. Preobrazhenskaya, Khim. Geterotsikl. Soedin., No. 11, 1556 (1984).
I. D. Shingarova, I. V. Yartseva, and M. N. Preobrazhenskaya, Khim. Geterotsikl. Soedin., No. 2, 231 (1987).
J. L. Aubagnac, P. Campion, and P. Guenot, Org. Mass Spectrom., 13, 571 (1978).
G. C. Levy and G. L. Nelson, Carbon-13 Nuclear Magnetic Resonance for Organic Chemists, (1972), pp. 107, 126.
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 7, pp. 937–940, July, 1987.
Rights and permissions
About this article
Cite this article
Shingarova, I.D., Lebedev, A.T. & Preobrazhenskaya, M.N. Direction of glycosylation of 5-substituted 4-chloro-1,2,3-triazoles. Chem Heterocycl Compd 23, 769–772 (1987). https://doi.org/10.1007/BF00475646
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00475646