Abstract
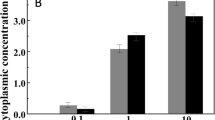
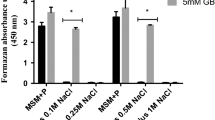
The accumulation of glycine betaine to a high internal concentration by Escherichia coli cells in high osmolarity medium restores, within 1 h, a subnormal growth rate. The experimental results support the view that cell adaptation to high osmolarity involves a decrease in the initiation frequency of DNA replication via a stringent response; in contrast, glycine betaine transport and accumulation could suppress the stringent response within 1–2 min and restore a higher initiation frequency. High osmolarity also triggers the cells to lengthen, perhaps via an inhibition of cellular division; glycine betaine also reverses this process. It is inferred that turgor could control DNA replication and cell division in two separate ways. Glycine betaine action is not mediated by K+ ions as the internal level of K+ ions is not modified significantly following glycine betaine accumulation.
Similar content being viewed by others
References
Barron A, May G, Bremer E, Villarejo M (1986) Regulation of envelope protein composition during adaptation to osmotic stress in Escherichia coli. J Bacteriol 167:433–438
Cairney J, Booth IR, Higgins CF (1985a) Osmoregulation of gene expression in Salmonella typhimurium: pro U encodes an osmotically induced betaine transport system. J Bacteriol 164:1224–1232
Cairney J, Booth IR, Higgins CF (1986b) Salmonella typhimurium pro P gene encodes a transport system for the osmoprotectant betaine. J Bacteriol 164:1088–1093
Carl PL (1970) Escherichia coli mutants with temperature-sensitive synthesis of DNA. Mol Gen Genet 109:107–122
Christian JGB (1955) The influence of nutrition on the water relations of Salmonella oranienburg. Aust J Biol Sci 8:75–82
Clark D, Parker J (1984) Proteins induced by high osmotic pressure in Escherichia coli. FEMS Microbiol Lett 25:81–83
Csonka LN (1981) Proline over-production results in enhanced osmotolerance in Salmonella typhimurium. Mol Gen Genet 182:82–86
Csonka LN (1982) A third l-proline permease in Salmonella typhimurium which functions in media of elevated osmotic strength. J Bacteriol 151:1433–1443
Epstein W, Schultz SG (1965) Cation transport in Escherichia coli. V. Regulation of cation content. J Gen Physiol 49:221–234
Gallant JA (1979) Stringent control in Escherichia coli. Ann Rev Genet 13:393–415
Girija R, Ikenaka K, Inouye M (1985) Uncoupling of osmoregulation of the Escherichia coli K-12 omp F gene from omp B-dependent transcription. J Bacteriol 163:82–87
Glass RE, Jones S, Ishihama A (1986) Genetic studies on the β subunit of Escherichia coli RNA polymerase. VII. RNA polymerase is a target for ppGpp. Mol Gen Genet 203:265–268
Gowrishankar Y (1985) Identification of osmo-responsive genes of Escherichia coli: evidence for participation of potassium and proline transport systems in osmoregulation. J Bacteriol 164:434–445
Hecker M, Schroeter A, Mach F (1983) Replication of pBR 322 DNA in stringent and relaxed strains of Escherichia coli. Mol Gen Genet 190:355–357
Jackson BJ, Kennedy EP (1983) The biosynthesis of membrane-derived oligosaccharides. A membrane-bound phosphoglycerol transferase. J Biol Chem 258:2394–2398
Kawaji H, Mizuno T, Mizushima S (1979) Influence of molecular size and osmolarity of sugars and dextrans on the synthesis of outer membrane proteins 0-8 and 0-9 of Escherichia coli K-12. J Bacteriol 140:843–847
Ken-Dror S, Preger R, Avi-Dor Y (1986) Role of betaine in the control of respiration and osmoregulation of a halotolerant bacterium. FEMS Microbiol Rev 39:115–120
Kennedy EP (1982) Osmotic regulation and the biosynthesis of membrane-derived oligosaccharides. Proc Natl Acad Sci USA 79:1092–1095
Koch A, Higgins MC, Doyle R (1981) Surface tension-like forces determine bacterial shapes: Streptococcus faecium. J Bacteriol 147:97–100
Koch A (1984) Shrinkage of growing Escherichia coli cells by osmotic challenge. J Bacteriol 159:919–924
Kogut M, Russel NJ (1984) Growth and phospholipid composition of a moderately halophilic bacterium during adaptation to changes in salinity. Curr Microbiol 10:95–98
Kubitschek HE, Freedman ML, Silver S (1971) Potassium uptake in synchronous and synchronized cultures of Escherichia coli. Biophys J 11:787–795
Laffler T, Gallant JA (1974) A new genetic locus involved in the stringent response in Escherichia coli. Cell 1:27–30
Lebail S (1979) Transport du K+ au cours du cycle cellulaire chez Escherichia coli. Thesis, Université de Paris
Legros M, Kepes A (1985) One-step fluorometric microassay of DNA in procaryotes. Anal Biochem 147:497–502
Le Rudulier D, Valentine RC (1982) Genetic engineering in agriculture: osmoregulation. TIBS 7:431–433
Le Rudulier D, Bouillard L (1983) Glycine betaine, an osmotic effector in Klebsiella pneumoniae and other members of the Enterobacteriaceae. Appl Environ Microbiol 46:152–159
Le Rudulier D, Strom AR, Dandekar AM, Smith LT, Valentine RC (1984) Molecular biology of osmoregulation. Science 224:1064–1068
Lin-Chao S, Bremer H (1986) Effect of rel A function on the replication of plasmid pBR322 in Escherichia coli. Mol Gen Genet 203:150–153
Lowry OH, Rosebrough NJ, Farr AL, Randal RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Meury J (1976) Potassium transport in Escherichia coli. Thesis, Université de Paris
Meury J, Robin A, Monier-Champex P (1985) Turgor-controlled fluxes and their pathways in Escherichia coli. Eur J Biochem 151:613–619
Miller KJ, Kennedy EP, Reinhold VN (1986) Osmotic adaptation by Gram-negative bacteria: possible role for periplasmic oligosaccharides. Science 231:48–51
Munro GF, Bell CA (1973) Effects of external osmolarity on phospholipid metabolism in Escherchiaa coli B. J Bacteriol 166:257–262
Olijhoek AJM, Van Eden CG, Trueba F, Pas E, Anninga N (1982) Plasmolysis during the division cycle of Escherichia coli. J Bacteriol 152:479–484
Perroud B, Le Rudulier D (1985) Glycine betaine transport in Escherichia coli. Osmotic modulation. J Bacteriol 161:393–401
Roth W, Leckie M, Dietzler D (1985a) Osmotic stress drastically inhibits active transport of carbohydrates by Escherichia coli. Biochem Biophys Res Commun 126:434–441
Roth W, Porter S, Leckie M, Porter B, Dietzler D (1985b) Restoration of cell volume and the reversal of carbohydrate transport and growth inhibition of osmotically upshocked Escherichia coli. Biochem Biophys Res Commun 126:442–449
Russel NJ, Kogut M (1985) Haloadaptation: salt sensing and cell-envelope changes. Microbiol Sci 11:345–350
Thiam K, Farve A (1984) Role of the stringent response in the expression and mechanism of near-ultraviolet induced growth delay. Eur J Biochem 145:137–142
Villarejo M, Davis J, Granett S (1983) Osmoregulation of Alkaline phosphatase synthesis in Escherichia coli K-12. J Bacteriol 156:975–978
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meury, J. Glycine betaine reverses the effects of osmotic stress on DNA replication and cellular division in Escherichia coli . Arch. Microbiol. 149, 232–239 (1988). https://doi.org/10.1007/BF00422010
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00422010