Summary
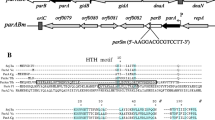
The seg mutants (seg-1 and seg-2) of Escherichia coli cannot support the replication of the F factor and mini-F plasmids at 42°C. We cloned the wild-type E. coli chromosomal DNA fragment complementing the seg-1 and seg-2 mutations and found that both mutations were complemented by the wild-type dnaK gene coding for a heat shock protein. Transduction with phage P1 indicated that the seg-2 mutation is located at about 0.3 min in the region containing the dnaK gene in the order trpR-thrA-seg-2-leuB, consistent with the locus of the dnaK gene. Cloning and sequencing of the dnaK gene of the seg mutants showed that there was one base substitution within the dnaK gene in each mutant causing an amino acid substitution. These results indicate that the seg gene in which the seg-1 and seg-2 mutations occurred is identical to the dnaK gene. The mini-F plasmid pXX325 did not transform a dnaK null mutant to ampicillin resistance at 30°C in contrast to plasmids pBR322, pACYC184 and pSC101, which did. The active dnaK (seg) gene product is therefore essential for replication of the mini-F plasmid at both 30° and 42°C.
Similar content being viewed by others
References
Bachmann BJ (1983) Linkage map of Escherichia coli K-12, Edition 7. Microbiol Rev 47:180–230
Bardwell JCA, Craig EA (1984) Major heat shock gene of Drosophila and the Escherichia coli heat-inducible dnaK gene are homologous. Proc Natl Acad Sci USA 81:848–852
Bex F, Karoui H, Rokeach L, Dréze P, Garcia L, Coutuier M (1983) Mini-F encoded proteins: identification of a new 10.5 kilodalton species. EMBO J 2:1853–1861
Bex F, Piérard P, Desmyter A, Dréze P, Colet M, Couturier M (1986) Mini-F E protein: the carboxy-terminal end is essential for E gene repression and mini-F copy number control. J Mol Biol 189:293–303
Bolivar F, Rodriguez RL, Greene PJ, Betlach MC, Heyneker HL, Boyer HW, Crosa JH, Falkow S (1977) Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95–113
Brady G, Jantzen HM, Bernard HU, Brown R, Schütz G, Hashimoto-Gotoh T (1984) New cosmid vectors developed for eukaryotic DNA cloning. Gene 27:223–232
Chang ACY, Cohen SN (1978) Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol 134:1141–1156
Cohen SN, Chang ACY, Boyer HW, Helling RB (1973) Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci USA 70:3240–3244
Frischauf AM, Lehrach H, Poustka A, Murray N (1983) Lambda replacement vectors carrying polylinker sequences. J Mol Biol 170:827–842
Hansen EB, Yarmolinksy MB (1986) Host participation in plasmid maintenance: dependence upon dnaA of replicons derived from P1 and F. Proc Natl Acad Sci USA 83:4423–4427
Hathaway BG, Bergquist PL (1973) Temperature-sensitive mutations affecting the replication of F-prime factors in Escherichia coli K12. Mol Gen Genet 127:297–306
Hayakawa Y, Murotsu T, Matsubara K (1985) Mini-F protein that binds to a unique region for partition of mini-F plasmid DNA. J Bacteriol 163:349–354
Hiraga S, Jaffé A, Ogura T, Mori H, Takahashi H (1986) F plasmid ccd mechanism in Escherichia coli. J Bacteriol 166:100–104
Itikawa H, Ryu J (1979) Isolation and characterization of a temperature-sensitive dnaK mutant of Escherichia coli B. J Bacteriol 138:339–344
Jaffé A, Ogura T, Hiraga S (1985) Effects of the ccd function of the F plasmid on bacterial growth. J Bacteriol 163:841–849
Jamieson AF, Bergquist PL (1976) Genetic mapping of chromosomal mutants affecting the replication of the F-factor of Escherichia coli. Mol Gen Genet 148:221–223
Jamieson AF, Bergquist PL (1977) Plasmid replication and Hfr formation in strains of Escherichia coli carrying seg mutations. Mol Gen Genet 150:171–181
Karoui H, Bex F, Dréze P, Couturier M (1983) Ham22, a mini-F mutation which is lethal to host cell and promotes recA-dependent induction of lambdoid prophage. EMBO J 2:1863–1868
Keller JA, Simon LD (1988) Divergent effects of a dnaK mutation on abnormal protein degradation in Escherichia coli. Mol Microbiol 2:31–41
Kline BC (1985) A review of mini-F plasmid maintenance. Plasmid 14:1–16
Kohara Y, Akiyama K, Isono K (1987) The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell 50:495–508
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning, a laboratory manual. Cold Spring Harbor Laboratory Press, NY
Messing J (1983) New M13 vectors for cloning. Methods Enzymol 101:20–78
Miki T, Yoshioka K, Horiuchi T (1984) Control of cell division by sex factor F in Escherichia coli. I. The 42.84–43.6 F segment couples cell division of the host bacteria with replication of plasmid DNA. J Mol Biol 174:605–625
Mori H, Kondo A, Ohshima A, Ogura T, Hiraga S (1986) Structure and function of the F plasmid genes essential for partition. J Mol Biol 192:1–15
Murakami Y, Ohmori H, Yura T, Nagata T (1987) Requirement of the Escherichia coli dnaA gene function for ori2-dependent mini-F plasmid replication. J Bacteriol 169:1724–1730
Niki H, Ichinose C, Ogura T, Mori H, Morita M, Hasegawa M, Kusukawa N, Hiraga S (1988) Chromosomal genes essential for stable maintenance of the mini-F plasmid in Escherichia coli. J Bacteriol 170:5272–5278
O'Connor MB, Kilbane JJ, Malamy MH (1986) Site-specific and illegitimate recombination in the oriV1 region of the F factor: DNA sequences involved in recombination and resolution. J Mol Biol 189:85–102
Ogura T, Hiraga S (1983a) Partition mechanism of F plasmid: two plasmid gene-encoded products and a cis-acting region are involved in partition. Cell 32:351–360
Ogura T, Hiraga S (1983b) Mini-F plasmid genes that couple host cell division to plasmid proliferation. Proc Natl Acad Sci USA 80:4784–4788
Ohki M, Tamura F, Nishimura S, Uchida H (1986) Nucleotide sequence of the Escherichia coli dnaJ gene and purification of the gene product. J Biol Chem 261:1778–1781
Paek K, Walker GC (1987) Escherichia coli dnaK null mutants are inviable at high temperature. J Bacteriol 169:283–290
Rokeach LA, Søgaard-Andersen L, Molin S (1985) Two function of the E protein are key elements in the plasmid F replication control system. J Bacteriol 164:1262–1270
Sakakibara Y (1988) The dnaK gene of Escherichia coli functions in initiation of chromosome replication. J Bacteriol 170:972–979
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Silhavy TJ, Berman ML, Enquist LW (1984) Experiments with gene fusions. Cold Spring Harbor Laboratory Press, NY
Stadler J, Adelberg EA (1972) Temperature dependence of sexfactor maintenance in Escherichia coli K-12. J Bacteriol 109:447–449
Takeshita S, Sato M, Toba M, Masahashi W, Hashimoto-Gotoh T (1987) High-copy-number and low-copy-number plasmid vectors for lacZ α-complementation and chloramphenicol- or kanamycin-resistance selection. Gene 61:63–74
Tilly K, McKittrick N, Zylicz M, Georgopoulos C (1983) The dnaK protein modulates the heat-shock response of Escherichia coli. Cell 34:641–646
Trawick JD, Kline BC (1985) A two-stage molecular model for control of mini-F replication. Plasmid 13:59–69
Tokino T, Murotsu T, Matsubara K (1986) Purification and properties of the mini-F plasmid-encoded E protein needed for autonomous replication control of the plasmid. Proc Natl Acad Sci USA 83:4109–4113
Wada C, Yura T (1982) Inhibition of initiation of mini-F plasmid replication in temperature-sensitive mafA mutants of Escherichia coli K-12. Plasmid 8:287–298
Wada C, Yura T (1984) Control of F plasmid replication by a host gene: evidence for interaction of the mafA gene product of Escherichia coli with the mini-F incC region. J Bacteriol 160:1130–1136
Wada C, Hiraga S, Yura T (1976) A mutant of Escherichia coli incapable of supporting vegetative replication of F-like plasmids. J Mol Biol 108:25–41
Wada C, Yura T, Hiraga S (1977) Replication of F poh + plasmid in mafA mutants of Escherichia coli defective in plasmid maintanence. Mol Gen Genet 152:211–217
Wada C, Imai M, Yura T (1987) Host control of plasmid replication: requirement for the δ factor δ32 in transcription of mini-F replication initiator gene. Proc Natl Acad Sci USA 84:8849–8853
Wada M, Kano Y, Ogawa T, Okazaki T, Imamoto F (1988) Construction and characterization of the deletion mutant of hupA and hupB genes in Escherichia coli. J Mol Biol 204:581–591
Yamagata H, Uchida H (1972) Spectinomycin resistance mutations affecting the stability of sex-factors in Escherichia coli. J Mol Biol 67:533–535
Yochem J, Uchida H, Sunshine M, Saito M, Georgopoulos CP, Feiss M (1978) Genetic analysis of two genes, dnaJ and dnaK, necessary for Escherichia coli and bacteriophage lambda DNA replication. Mol Gen Genet 164:9–14
Zylicz M, Georgopoulos C (1984) Purification and properties of the Escherichia coli dnaK replication protein. J Biol Chem 259:8820–8825
Zylicz M, LeBowitz JH, McMacken R, Georgopoulos C (1983) The dnaK protein of Escherichia coli possesses an ATPase and autophosphorylating activity and is essential in an in vitro DNA replication system. Proc Natl Acad Sci USA 80:6431–6435
Author information
Authors and Affiliations
Additional information
Communicated by M. Sekiguchi
Rights and permissions
About this article
Cite this article
Ezaki, B., Ogura, T., Mori, H. et al. Involvement of DnaK protein in mini-F plasmid replication: Temperature-sensitive seg mutations are located in the dnaK gene. Molec Gen Genet 218, 183–189 (1989). https://doi.org/10.1007/BF00331267
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00331267