Abstract
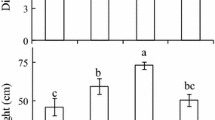
Photosynthetic induction of in situ saplings of two Costa Rican rainforest tree species wre compared in relation to their light environment, using infrared gas analysis and hemispherical photography. The species studied were Dipteryx panamensis, a climax species found in bright microsites, and Cecropia obtusifolia, a pioneer species. In the morning, when leaves were most responsive, induction time necessary to reach 90% of the lightsaturated rate of photosynthesis was on average 16 min for Dipteryx and 10 min for Cecropia. However, induction times for both species increased in the afternoon resulting in shorter daily average induction times for Dipteryx than for Cecropia. Dipteryx also maintained higher levels of induction for a longer period under low light conditions than did Cecropia. The two species differed in the way they adjusted to light availability. Dipteryx saplings growing in shady sites had faster rates of induction than saplings growing in bright sites, with no difference in light-saturated photosynthetic rate. In contrast, Cecropia saplings growing in bright sites had higher light-saturated photosynthetic rates than saplings growing in shady sites, with no difference in rates of induction. Dipteryx appears to exploit temporal variation in light availability by refining the quickness of the induction response to the light environment, while Cecropia adjusts its scale of exploitation by realizing a higher lightsaturated photosynthetic rate in sites of higher light.
Similar content being viewed by others
References
Alvey N, Galwey N, Lane P (1982) An introduction to GENSTAT. Academic, London
Bazzaz FA, Carlson RW (1982) Photosynthetic acclimation to variability in the light environment of early and late successional plants. Oecologia 54: 313–316
Chazdon R (1988) Sunflecks and their importance to forest understorey plants. Adv Ecol Res 18: 1–63
Chazdon RL, Fetcher N (1984) Photosynthetic light environments in a lowland tropical rain forest of Costa Rica. J Ecol 72: 553–564
Chazdon RL, Field CB (1987) Determinants of photosynthetic capacity in six rainforest Piper species. Oecologia 73: 222–230
Chazdon RL, Pearcy RW (1986) Photosynthetic responses to light variation in rainforest species. I. Induction under constant and fluctuating light conditions. Oecologia 69: 517–523
Clark DB, Clark DA (1987a) Population ecology and microhabitat distribution of Dipteryx panamensis, a Neotropical rain forest emergent tree. Biotropica 19: 236–244
Clark DA, Clark DB (1987b) Analisis de la regeneración de árboles del dosel en bosque muy húmedo tropical: aspectos teóreticos y prácticos. Rev Biol Trop 35 (Suppl): 41–54
Clark DA, Clark DB (1992) Life history diversity of canopy and emergent trees in a Neotropical rain forest. Ecol Monogr 62: 315–344
Edwards G, Walker D (1983) C3, C4: mechanisms, and cellular and environmental regulation, of photosynthesis, University of California Press, berkeley
Hartshorn G (1983) Plants. In: Janzen DH (ed) Costa Rican natural history. University of Chicago Press, Chicago, pp 118–157
Hunt R, Hand DW, Hannah MA, Neal AM (1991) Response to CO2 enrichment in 27 herbaceous species. Funct Ecol 5: 410–421
Kirschbaum MUF, Pearcy RW (1988a) Gas exchange analysis of the fast phase of photosynthetic induction in Alocasia macrorrhiza. Plant Physiol 87: 818–821
Kirschbaum MUF, Pearcy RW (1988b) Gas exchange analysis of the relative importance of stomatal and biochemical factors in photosynthetic induction in Alocasia macrorrhiza. Plant Physiol 86: 782–785
Küppers M, Schneider H (1993) Leaf gas exchange of beech (Fagus sylvatica L.) seedlings in lightflecks: effects of fleck length and leaf temperature in leaves grown in deep and partial shade. Trees 7: 160–168
Kursar TA, Coley PD (1993) Photosynthetic induction times in shade tolerant species with long and short-lived leaves. Oecologia 93: 165–170
Oberbauer SF (1985) Plant water relations of selected species in wet and dry tropical lowland forests in costa Rica. Rev Biol Trop 33: 137–142
Oberbauer SF, Clark DB, Clark DA, Quesada M (1988) Crown light environments of saplings of two species of rain forest emergent trees. Oecologia 75: 207–212
Pearcy RW (1983) The light environment and growth of C3 and C4 tree species in the understory of a Hawaiian forest. Oecologia 58: 19–25
Pearcy RW (1987) Photosynthetic gas exchange responses of Australian tropical forest trees in canopy, gap and understory micro environments. Funct Ecol 1: 169–178
Pearcy RW (1989) Regulation of photosynthetic CO2 assimilation during sunflecks. In: Briggs RW (ed) Photosynthesis. Alan R Liss, New York, pp 407–424
Pearcy RW (1990) Sunflecks and photosynthesis in plant canopies. Annu Rev Physiol Plant Mol Biol 41: 421–453
Pearcy RW, Schulze ED, Zimmermann R (1989) Measurement of transpiration and leaf conductance. In: Pearccy RW, Ehleringer JR, Mooney HA, Runder PW (eds). Plant physiological ecology. Field methods and instrumentation. Chapman and Hall, London, pp 137–160
Pfitsch WA, Pearcy RW (1989) Steady-state and dynamic photosynthetic response of Adenocaulon bicolor (Asteraceae) in its redwood forest habitat. Oecologia 80: 471–476
Rich PM (1989) A manual for analysis of hemispherical canopy photography. (Technical manual LA-11733-M). National Technical Information Service, Springfield
Riddoch I, Grace J, Fesahun FE, Riddoch B, Lapidos DO (1991) Photosynthesis and successional status of seedlings in a tropical semi-deciduous rain forest in Nigeria. J Ecol 79: 491–503
Seemann JR, Kirschbaum MUF, Sharkey TD, Pearcy RW (1988) Regulation of ribulose bisphosphate carboxylase activity in Alocasia macrorrhiza in response to step changes in irradiance. Plant Physiol 88: 148–152
Sharkey TD (1985) Photosynthesis in intact leaves of C3 plants: physics, physiology and rate limitations. Bot Rev 51: 53–105
SPSS (1990) SPSS/PC+V4.0 Base manual. SPSSINC, Chicago
Woodrow IE, Mott KA (1989) Rate limitation of non-steady-state photosynthesis by ribulose-1,5-biphosphate carboxylase in spinach. Aust J Plant Physiol 16: 487–500
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Poorter, L., Oberbauer, S.F. Photosynthetic induction responses of two rainforest tree species in relation to light environment. Oecologia 96, 193–199 (1993). https://doi.org/10.1007/BF00317732
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00317732