Abstract
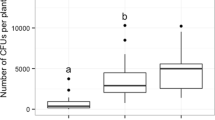
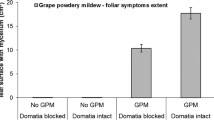
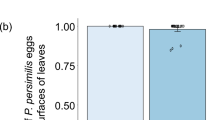
Associations between mites and leaf domatia have been widely reported, but little is known about their consequences for either plants or mites. By excising domatia from leaves of the laureltinus, Viburnum tinus L. (Caprifoliaceae), in the garden and laboratory, we showed that domatia alter the abundance, distribution, and reproduction of potential plant mutualists. Over 4 months, leaves with domatia on six garden shrubs had 2–36 times more predatory and microbivorous mites, and more mite eggs than leaves without domatia. However, this effect varied among plants and was weaker on one shrub with few mites on its leaves. Domatia also influenced the distribution of mites on leaves. A significantly higher fraction of mites, representing all life stages, was found in vein axils of leaves with domatia than in vein axils on leaves without domatia. Single-leaf experiments in the laboratory showed that domatia enhanced reproduction by the predatory mite, Metaseiulus occidentalis, especially at low relative humidity (30–38%). When domatia were removed, oviposition was reduced significantly only at low relative humidity, suggesting that domatia provide mites with refuge from environmental extremes on the leaf surface. Moreover, the use of domatia by predatory mites may reduce the impact of some plant enemies. In two experiments where prey consumption was measured, M. occidentalis ate significantly higher percentages of the eggs of the two-spotted spider mite (Tetranychus urticae). Our results are consistent with the viewpoint that mite-domatia associations are mutualistic. By directly aiding and abetting the third trophic level, plants with leaf domatia may increase the efficiency of some predaceous and microbivorous mites in consuming plant enemies.
Similar content being viewed by others
References
Altieri MA, Lewis WJ, Nordlund RC, Gueldner RC, Todd JW (1981) Chemical interactions between plants and Trichogramma wasps in Georgia soybean fields. Prot Ecol 3:259–263
Bailey LH, Bailey EZ (1978) Hortus third: a concise dictionary of plants cultivated in the United States and Canada. Macmillan, New York
Badii MH, McMurtry JA (1984) Life history of and life table parameters for Phytoseiulus longipes with comparative studies on P. persimilis and Typhlodromus occidentalis (Acari: Phytoseiidae). Acarologica 25:111–123
Bakker FM, Klein ME, Mesa NC, Braun AR (1993) Saturation deficit tolerance spectra of phytophagous mites and their phytoseiid predators on cassava. Exp Appl Acarol 17:97–113
Barbosa P, Letourneau DK (1988) Novel aspects of insect-plant interactions. Wiley, New York
Chant DA, Muir RC (1955) A comparison of the imprint and brushing machine methods for estimating the numbers of fruit tree red spider mite, Metatetranychus ulmi (Koch), on apple leaves. Rep E Malling Res Sta 141–145
Croft BA, Messing RH, Dunley JE, Strong WB (1993) Effects of humidity on eggs and immatures of Neoseiulus fallacis, Amblyseius andersoni, Metaseiulus occidentalis and Typhlodromus pyri (Phytoseiidae): implications for biological control on apple, caneberry, strawberry and hop. Exp Appl Acarol 17:451–459
Dicke M, Sabelis MW (1988) How plants obtain predatory mites as bodyguards. Neth J Zool 38:148–165
Dinh NV, Sabelis MW, Janssen A (1988) Influence of humidity and water availability on the survival of Amblyseius idaeus and A. anonymus (Acarina: Phytoseiidae). Exp Appl Acarol 4:27–40
Downing RS, Moilliet TK (1967) Relative densities of predacious and phytophagous mites on three varieties of apple trees. Can Entomol 99:738–741
Duso C (1992) Role of Amblyseius aberrans (Oud.), Typhlodromus pyri Scheuten and Amblyseius andersoni (Chant) (Acari, Phytoseiidae) in vineyards. III. Influence of variety characteristics on the success of A. aberrans and T. pyri releases. J Appl Entomol 114:455–462
Ehleringer JR (1984) Ecology and ecophysiology of leaf pubescence in North American desert plants. In: Rodriguez E, Healey P, Mehta I (eds) Biology and chemistry of plant trichomes. Plenum, New York, pp 113–132
Ferro DN, Southwick EE (1984) Micro-climates of small arthropods: estimating humidity within the leaf boundary layer. Environ Entomol 13:926–929
Friese DD, Gilstrap FE (1982) Influence of prey availability on reproduction and prey consumption of Phytoseiulus persimilis, Amblyseius californicus and Metaseiulus occidentalis (Acarina: Phytoseiidae). Int J Acarol 8:85–89
Flaherty DL, Huffaker CB (1970) Biological control of Pacific mites and Willamette mites. I. Role of Metaseiulus occidentalis. Hilgardia 40:267–308
Fleschner CA, Arakawa KY (1952) The mite Tydeus californicus on citrus and avocado leaves. J Econ Entomol 45: 1092
Gaede K (1992) On the water balance of Phytoseiulus persimilis A.-H. and its ecological significance. Exp Appl Acarol 15:181–198
Jacobs M (1966) On domatia — the viewpoints and some facts. 1. Acad Weten Amsterdam 69:275–316
Jeppson LR, Keifer HH, Baker EW (1975) Mites injurious to economic plants. Univesity of California Press, Berkeley
Krantz GW (1978) A manual of acarology, 2nd edn. Oregon State University Book Stores, Corvallis, Oregon
Lenteren JC van, Ponti OMB de (1990) Plant-leaf morphology, host-plant resistance and biological control. Symp Biol Hung 39: 365–386
Lundströem AN (1887) Von Domatien. Pflanzenbiologische Studien. II. Die Anpassung der Pflanzen und Thiere. Nova Acta Regiae Soc Sci Ups Ser 3 XIII, pp 1–87
McMurtry JA, Scriven GT (1965) Insectary production of phytoseiid mites. J Econ Entomol 58:282–284
O'Dowd DJ, Willson MF (1989) Leaf domatia and mites on Australasian plants: ecological and evolutionary implications. Biol J Linn Soc 37:191–236
O'Dowd DJ, Willson MF (1991) Associations between mites and leaf domatia. Trend Ecol Evol 6:173–200
Overmeer WPJ, Zon AQ van (1984) The preferences of Amblyseius potentillae (Acarina: Phytoseiidae) for certain plant substrates. In: Griffiths DA, Bowman CE (eds) Acarology IV. vol 1. Horwood, Chichester, pp 591–596
Pemberton RW, Turner CE (1989) Occurrence of predatory and fungivorous mites in leaf domatia. Am J Bot 76:105–112
Penzig O, Chiabrera C (1903) Contributo alla conoscenza delle piante acarofile. Malphigia 17:429–487, plates 16–18
Pillemer EA, Tingey WM (1976) Hooked trichomes: a physical plant barrier to a major agricultural pest. Science 193: 482–484
Price PW, Bouton CE, Gross P, McPheron BA, Thompson JN, Weis AE (1980) Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu Rev Ecol Syst 11:41–65
Sabelis M (1985) Development. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control, vol 1A. Elsevier, Amsterdam, pp 43–53
Schicha E (1978) The morphology of three Typhlodromus occidentalis (Nesbitt) populations in Australia (Acarina: Phytoseiidae). Z Angew Zool 65:291–302
Schnell R, Cusset G, Tchinaye V, Tô NA (1968) Contribution à l'étude des “Acarodomaties”. La question des asselles de nervures. Rev Gen Bot 75:5–64
Sokal RR, Rohlf FJ (1981) Biometry, 2 edn. WH Freeman, San Francisco
Solomon ME (1951) Control of humidity with potassium hydroxide, sulfuric acid and other solutions. Bull Entomol Res 42:543–554
Steenis WJJJ van (1976) Autonomous evolution in plants. Gard Bull Singapore 29:103–126
Swift FC, Blaustein L (1980) Humidity tolerances of 3 species of phytoseiid mites (Acarina: Phytoseiidae). Ann NY Entomol Soc 88:77
Takabayashi J, Dicke M, Posthumus MA (1991) Variation in composition of predator-attracting allelochemicals emitted by herbivore-infested plants: relative influence of plant and herbivore. Chemoecology 2:1–6
Tomczyk A, Kropczynska D (1985) Effects of the host plant. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control, vol 1A. Elsevier, Amsterdam, pp 317–327
Walter DE (1992) Leaf surface structure and the distribution of Phytoseius mites (Acarina: Phytoseiidae) in south-eastern Australian forests. Aust J Zool 40:593–603
Walter DE, Denmark HA (1991) Use of leaf domatia on wild grape (Vitis munsoniana) by arthropods in central Florida. Fl Entomol 74:440–446
Walter DE, O'Dowd DJ (1992a) Leaves with domatia have more mites. Ecology 73:1514–1518
Walter DE, O'Dowd DJ (1992b) Leaf morphology and predators: effect of leaf domatia on the abundance of predatory mites (Acari: Phytoseiidae). Environ Entomol 21:478–484
Walter DE, O'Dowd DJ (in press) Life on the forest phylloplane: hairs, little houses, and myriad mites. In: Lowman ME, Nadkarni N (eds) The forest canopy: aspects of research on this biological frontier. Academic Press, New York
Willmer PG (1986) Microclimatic effects on insects at the plant surface. Juniper B, Southwood R (eds). Insects and the plant surface. Edward Arnold, London, pp 65–80
Willson MF (1991) Foliar shelters for mites in the eastern deciduous forest. Am Midl Nat 126:111–117
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grostal, R., O'Dowd, D.J. Plants, mites and mutualism: leaf domatia and the abundance and reproduction of mites on Viburnum tinus (Caprifoliaceae). Oecologia 97, 308–315 (1994). https://doi.org/10.1007/BF00317319
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00317319