Abstract
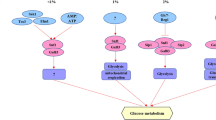
Yeast cells defective in the GGS1 (FDP1/BYP1) gene are unable to adapt to fermentative metabolism. When glucose is added to derepressed ggs1 cells, growth is arrested due to an overloading of glycolysis with sugar phosphates which eventually leads to a depletion of phosphate in the cytosol. Ggs1 mutants lack all glucose-induced regulatory effects investigated so far. We reduced hexokinase activity in ggs1 strains by deleting the gene HXK2 encoding hexokinase PII. The double mutant ggs1Δ, hxk2Δ grew on glucose. This is in agreement with the idea that an inability of the ggs1 mutants to regulate the initiation of glycolysis causes the growth deficiency. However, the ggs1Δ, hxk2Δ double mutant still displayed a high level of glucose-6-phosphate as well as the rapid appearance of free intracellular glucose. This is consistent with our previous model suggesting an involvement of GGS1 in transport-associated sugar phosphorylation. Glucose induction of pyruvate decarboxylase, glucoseinduced cAMP-signalling, glucose-induced inactivation of fructose-1,6-bisphosphatase, and glucose-induced activation of the potassium transport system, all deficient in ggs1 mutants, were restored by the delection of HXK2. However, both the ggs1Δ and the ggs1Δ, hk2Δ mutant lack detectable trehalose and trehalose-6-phosphate synthase activity. Trehalose is undetectable even in ggs1Δ strains with strongly reduced activity of protein kinase A which normally causes a very high trehalose content. These data fit with the recent cloning of GGS1 as a subunit of the trehalose-6-phosphate synthase/phosphatase complex. We discuss a possible requirement of trehalose synthesis for a metabolic balance of sugar phosphates and free inorganic phosphate during the transition from derepressed to fermentative metabolism.
Similar content being viewed by others
References
Bergmeyer HU (1974) Methods of enzymatic analysis, 2nd edn. Academic Press, New York
Breitenbach-Schmitt I, Schmitt HD, Heinisch J, Zimmermann FK (1984) Mol Gen Genet 195:536–540
Cannon JF, Morcos PA, Clemens KE, Khali M (1992) Yeast 8 (special issue): S359
Chapman C, Bartley W (1968) Biochem J 107:455–465
Chester V (1968) J Gen Microbiol 51:49–56
Duntze W, Neumann D, Holzer H (1968) Eur J Biochem 3:326–331
Eilam Y, Othman M, Halachmi D (1990) J Gen Microbiol 136:2537–2543
Entian KD (1986) Microbiol Sci 3:366–371
Farkas I, Hardy TA, Goebl MG, Roach PJ (1991) J Biol Chem 266:15602–15607
Francois J, Van Schaftingen E, Hers HG (1984) Eur J Biochem 145:187–193
Francois J, Van Schaftingen E, Hers HG (1988) Eur J Biochem 171:599–608
Francois J, Neves MJ, Hers HG (1991) Yeast 7:575–587
Fraenkel DG (1985) Proc Natl Acad Sci USA 87:4740–4744
Frascotti G, Baroni D, Martegani E (1990) FEBS Lett 274:19–22
Gancedo C (1971) J Bacteriol 107:401–405
Gancedo C, Schwerzmann K (1976) Arch Microbiol 109:221–225
Gancedo C, Serrano R (1989) In: Rose AH, Harrison JS (eds), The Yeasts vol 3, 2nd edn. Academic Press, New York, pp 205–259
Gancedo JM (1992) Eur J Biochem 206:297–313
Gancedo JM, Gancedo C (1971) Arch Microbiol 76:132–138
Gonzáles MI, Stucka R, Blázquez MA, Feldmann H, Gancedo C (1992) Yeast 8:183–192
Görts CPM (1969) Biochim Biophys Acta 184:299–305
Hohmann S, Huse K, Valentin E, Mbonyi K, Thevelein JM, Zimmermann FK (1992) J Bacteriol 174:4183–4188
Hottiger T, Schmutz P, Wiemken A (1987a) J Bacteriol 169:5518–5522
Hittiger T, Boller T, Wiemken A (1987b) FEBS Lett 220:113–115
Hottiger T, Boller T, Wiemken A (1989) FEBS Lett 255:431–434
Kaibuchi K, Miyajima A, Arai K, Matsumoto K (1986) Proc Natl Acad Sci USA 83:8172–8176
Koning W de, Van Dam K (1992) Anal Biochem 204:118–123
Lillie SH, Pringle JR (1980) J Bacteriol 143:1384–1394
Lobo Z, Maitra PK (1977) Genetics 86:727–744
Londesborough J, Vuorio O (1991) J Gen Microbiol 137:323–330
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) J Biol Chem 193:265–275
Ma H, Botstein D (1986) Mol Cell Biol 6:4046–4052
Maitra PK, Lobo Z (1971) J Biol Chem 246:475–488
Maitra PK, Lobo Z (1983) Genetics 105:501–515
Marshall-Carlson L, Neigeborn L, Coons D, Bisson L, Carlson M (1991) Genetics 128:505–512
Matern H, Holzer H (1977) J Biol Chem 252:6399–6402
Moore PA, Sagliocco FA, Wood RMC, Brown AJP (1991) Mol Cell Biol 11:5330–5337
Navon G, Shulman RG, Yamano T, Eccleshall TR, Lam KB, Baranofski JJ, Marmur J (1979) Biochemistry 18:4487–4499
Neves MJ, Jorge JA, Francois JM, Terenzi HF (1991) FEBS Lett 283:19–22
Nikawa J, Cameron S, Toda T, Ferguson KW, Wigler M (1987) Genes Dev 1:931–937
Panek AC, de Araujo PS, Neto VM, Panek AD (1987) Curr Genet 11:459–465
Panek AC, Francois J, Panek AD (1988) Curr Genet 13:15–20
Ramos J, Szkutnicka K, Cirillo VP (1988) J Bacteriol 170:5375–5377
Ramos J, Haro R, Alijo R, Rodriguez-Navarro A (1992) J Bacteriol 174:2025–2027
Rose M, Albig W, Entian K-D (1991) Eur J Biochem 199:511–518
Schaaff I, Green JBA, Gozalbo D, Hohmann S (1989) Curr Genet 15:75–81
Schmitt HD, Zimmermann FK (1982) J Bacteriol 151:1146–1152
Schmitt HD, Ciriacy M, Zimmermann FK (1983) Mol Gen Genet 192:247–252
Serrano R (1983) FEBS Lett 156:11–14
Sherman F, Fink GR, Hicks JB (1986) Methods in yeast genetics, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Tatchell K, Robinson L, Breitenbach M (1985) Proc Natl Acad Sci USA 82:3785–3789
Thevelein JM (1984) Microbiol Rev 48:42–59
Thevelein JM (1991) Mol Microbiol 5:1301–1307
Thevelein JM (1992) Ant van Leeuwenhoek 62:109–130
Thevelein JM, Beullens M, Honshoven F, Hoebeeck G, Detremerie K, den Hollander JA, Jans AWH (1987) J Gen Microbiol 133:2191–2196
Thomas BJ, Rothstein R (1989) Cell 56:619–630
Toda T, Uno I, Ishikawa T, Powers S, Kataoka T, Broek D, Cameron S, Broach J, Matsumoto K, Wigler M (1985) Cell 40:27–36
Van Aelst L, Hohmann S, Zimmermann FK, Jans AWH, Thevelein JM (1991) EMBO J 10:2095–2104
Van de Poll KW, Schamhart DHJ (1977) Mol Gen Genet 154:61–66
Van de Poll KW, Kerkenaar A, Schamhart DHJ (1974) J Bacteriol 117:965–970
Vandercammen A, Francois J, Hers H-G (1989) Eur J Biochem 182:613–620
Vuorio O, Londesborough J, Kalkkinen N (1992) Yeast 8 (special issue): S626
Zamenhoff S (1957) Methods Enzymol 3:696–704
Author information
Authors and Affiliations
Additional information
Communicated by K. Wolf
Rights and permissions
About this article
Cite this article
Hohmann, S., Neves, M.J., de Koning, W. et al. The growth and signalling defects of the ggs1 (fdp1/byp1) deletion mutant on glucose are suppressed by a deletion of the gene encoding hexokinase PII. Curr Genet 23, 281–289 (1993). https://doi.org/10.1007/BF00310888
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00310888