Abstract
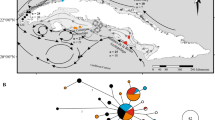
Allozyme variation at four loci and phenetic variation for esterase were examined in M. vertebralis populations from 10 reefs from the Western Coral Sea and two from the Great Barrier Reef (GBR). Genetic distances (Nei's D) among populations on different reefs ranged from 0–0.932 and was neither related to geographical separation of reefs nor to depth of water separating reefs. These findings suggest long-distance dispersal by some means is sufficient to prevent genetic differentiation of M. vertebralis populations, and that M. vertebralis populations need not be connected by habitats suitable for the continued existence of the foraminiferan for genetic differentiation to be prevented. The Western Coral Sea reef populations did not form a related group that were genetically distinct from those on the GBR but were differentiated latitudinally. Reefs to the extreme north and south formed outliers while those on the northern half of the Queensland Plateau showed some differentiation from those on the southern half of the Plateau. This pattern of genetic variation appeared to reflect the distribution of populations north and south of the southern limit of the Southern Equatorial Current. Further work will be required to establish the soundness of this relationship, and to exclude other possible explanations related to historical events or the effects of selection. Relatively high dispersal was inferred between the Southern Queensland Plateau reefs and those sampled on the GBR (average Neis D=0.011). Holmes and Marion reefs formed discrete genetic outliers (average Neis D=0.69 and 0.20 respectively). In the case of Holmes reef other factors (e.g. history of recruitment) will need to be investigated to account for its marked genetic differentiation from the other reefs in the Queensland Plateau.
Similar content being viewed by others
References
Andrews JC, Clegg S (1989) Coral Sea circulation and transport deduced from modal information models. Deep-Sea Res 36:957–974
Belbin L (1987) PATN pattern analysis package. CSIRO Division of Wildlife and Rangelands Research. Canberra, 299pp
Benzie JAH, Pandolfi JM (1991) Allozyme variation in M. vertebralis (Foraminifera: Miliolidae) from coral reef habitats in the Great Barrier Reef, Australia. J Foram Res (in press)
Church JA (1987) East Australian Current adjacent to the Great Barrier Reef. Aust J Mar Freshwat Res 38:671–683
Eanes WF, Koehn RK (1978) An analysis of genetic structure in the monarch butterfly, Danaus plexippus L. Evolution 32:784–797
Harris H, Hopkinson DA (1976) Handbook for enzyme electrophoresis in human genetics. North Holland, Oxford Looseleaf
Loeblich AR, Tappan H (1964) Protista 2, chiefly Thecamoebians and Foraminiferida. In: Moore RE (ed) Treatise of invertebrate palaeontology, part C, col 2. Geological Society of America and University of Kansas Press, Lawrence, 510 pp
Maxwell WGH (1968) Atlas of the Great Barrier Reef. Elsevier, Amsterdam, 258 pp
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Pickard GL, Donguy JR, Henin C, Rougerie F (1977) A review of the physical oceanography of the Great Barrier Reef and western Coral Sea. Aust Inst Mar Sci Monogr Ser 2:1–134
Richardson BJ, Baverstock PR, Adams M (1986) Allozyme electrophoresis. A handbook for animal systematics and population studies. Academic Press, Sydney, 410 pp
Ross CA (1972) Biology and ecology of Marginopora vertebralis (Foraminiferida), Great Barrier Reef. J Protozool 19:181–192
Shaklee JB, Keenan CP (1986) A practical laboratory guide to the techniques and methodology of electrophoresis and its application to fish fillet identification. CSIRO Marine Laboratories Report 177. CSIRO, Hobart, 59 pp
Stoddart JA (1983) Asexual production of planulae in the reef coral Pocillopora damicornis. Mar Biol 76:279–284
Stoddart JA (1988) Foliose Dictyoceratida of the Australian Great Barrier Reef III. Preliminary electrophoretic systematics. PSZNI: Mar Ecol 10:167–178
Swofford DL, Selander RB (1981) BIOSYS-1: A fortran program for the comprehensive analysis of electrophoretic data in population genetics and systematics. J Hered 72:281–283
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Benzie, J.A.H. Genetic relatedness of foraminiferan (Marginopora vertebralis) populations from reefs in the Western Coral Sea and Great Barrier Reef. Coral Reefs 10, 29–36 (1991). https://doi.org/10.1007/BF00301904
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00301904