Summary
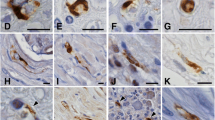
This report concerns the immunohistochemical demonstration of two neuronal Ca2+-binding proteins, calcineurin and synaptophysin, in the spinal cord of normal controls and from patients with familial dysautonomia. In controls, calcineurin immunoreactivity was highly concentrated in small nerve cells and fibers of the substantia gelatinosa. Synaptophysin immunoreactivity was normally distributed throughout the spinal cord gray matter, being highly concentrated in the substantia gelatinosa, the dorsal nucleus of Clarke and the anterior horn. In patients with familial dysautonomia, no apparent changes in calcineurin immunoreactivity were found in the substantia gelatinosa. By contrast, there was a significant depletion of synaptophysin-positive axon terminals in the substantia gelatinosa and in the dorsal nucleus of Clarke of patients with familial dysautonomia.
Similar content being viewed by others
References
Aguayo AJ, Nair CPV, Bray GM (1971) Peripheral nerve abnormalities in the Riley-Day syndrome, findings in a sural nerve biopsy. Arch Neurol 24:106–116
Bodian D (1975) Origin of specific synaptic types on the motoneuron neuropil of the monkey. J Comp Neurol 159:225–244
Carpenter MB, Sutin J (1983) Human neuroanatomy, 8th edn. Williams & Wilkins, Baltimore; pp 232–264
Conradi S, Kellerth J-O, Berthold C-H (1979) Electron microscopic studies of serially sectioned cat spinal alphamotoneurons. II. A method for the description of architecture and synaptology of the cell body and proximal dendritic segments. J Comp Neurol 184:741–754
Dyck PJ, Kawamura Y, Low PA, Shimono M, Solovy JS (1978) The number and sizes of reconstructed peripheral autonomic, sensory and motor neurons in a case of dysautonomia. J Neuropathol Exp Neurol 37:741–755
Goto S, Matsukado Y, Miyamoto E, Yamada M (1987) Morphological characterization of the rat striatal neurons expressing calcineurin immunoreactivity. Neuroscience 22:189–201
Goto S, Matsukado Y, Uemura S, Mihara Y, Inoue N, Ikeda J, Miyamoto E (1988) A comparative immunohistochemical study on calcineurin and S-100 protein in mammalian and avian brains. Exp Brain Res 69:645–650
Goto S, Hirano A, Rojas-Corona RR (1989) An immunohistochemical investigation of the human neostriatum in Huntington's disease. Ann Neurol 25:298–304
Goto S, Hirano A, Rojas-Corona RR (1989) Immunohistochemical visualization of afferent nerve terminals in human globus pallidus and its alteration in neostriatal neurodegenerative disorders. Acta Neuropathol 78:543–550
Goto S, Hirano A, Rojas-Corona RR (1989) A comparative immunohistochemical study of human cerebellar cortex in X-chromosome-liked copper malabsorption (Menkes' kinky hair disease) and granule cell type cerebellar degeneration. Neuropathol Appl Neurobiol 15:419–431
Hunt SP (1983) Cytochemistry of the spinal cord. In: Emson PC (ed) Chemical neuroanatomy. Raven Press, New York, pp 53–84
Inagaki S, Kito S (1986) Peptides in the peripheral nervous system. Prog Brain Res 66:269–316
Klee CB, Krinks MH, Manalan AS, Cohen P, Stewart AA (1983) Isolation and characterization of bovine calcineurin: a calmodulin-stimulated protein phosphatase. Methods Enzymol 102:227–244
Navone F, Jahn R, DiGioia G, Stukenbrok H, Greengard P, DeCamilli P (1986) Protein p38: an integral membrane protein specific for small vesicles of neurons and neuroendocrine cells. J Cell Biol 103:2511–2527
Pearson J, Budzilovich G, Finegold MJ (1971) Sensory, motor, and autonomic dysfunction: the nervous system in familial dysautonomia. Neurology 21:486–493
Pearson J, Axelrod F, Dancis J (1974) Current concepts of dysautonomia: neuropathological defects. Ann NY Acad Sci 228:288–300
Pearson J, Dancis J, Axelrod F, Grover N (1975) The sural nerve in familial dysautonomia. J Neuropathol Exp Neurol 34:413–424
Pearson J, Pytel BA, Grober-Johnson N, Axelrod F, Dancis J (1978) Quantitative studies of dorsal root ganglia and neuropathologic observations on spinal cords in familial dysautonomia. J Neurol Sci 35:77–92
Pearson J, Brandeis L, Cuello AC (1982) Depletion of substance P-containing axons in substantia gelatinosa of patients with diminished pain sensitivity. Nature 295:61–63
Rehm H, Wiedenmann B, Betz H (1986) Molecular characterization of synaptophysin, a major calcium-binding protein of the presynaptic vesicle membrane. EMBO J 5:535–541
Riley CM (1974) Familial dysautonomia: clinical and pathophysiological aspects. Ann NY Acad Sci 228:283–287
Riley CM, Day RL, Greeley DM, Langford WS (1949) Central autonomic dysfunction with defective lacrimation. I. Report of five cases. Pediatrics 3:468–478
Schwartz JP, Breakefield XO (1980) Altered nerve growth factor in fibroblasts from patients with familial dysautonomia. Proc Natl Acad Sci USA 77:1154–1158
Wiedenmann B, Franke WW (1985) Identification and localization of synaptophysin, an integral membrane glycoprotein of M r 38,000 characteristic of presynaptic vesicles. Cell 41:1017–1028
Womack MD, MacDermott AB, Jessell TM (1988) Sensory transmitters regulate intracellular calcium in dorsal horn neurons. Nature 334:351–353
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Goto, S., Hirano, A. & Pearson, J. Calcineurin and synaptophysin in the human spinal cord of normal individuals and patients with familial dysautonomia. Acta Neuropathol 79, 647–652 (1990). https://doi.org/10.1007/BF00294243
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00294243