Summary
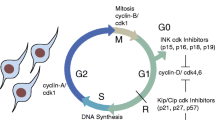
Neuroepithelial cells transform from spindle-shaped to wedge-shaped within the median and paired dorsolateral hinge points of the bending neural plate, but the mechanisms underlying these localized changes are unclear. This study was designed to evaluate further the hypothesis that localized “wedging” of neuroepithelial cells during bending involves basal cellular expansion resulting from alteration of the cell-cycle. Neurulating chick embryos were treated with tritiated thymidine, and transverse sections through the midbrain were examined autoradiographically. Parameters of the cell-cycle as well as nuclear position and size were assessed in the median hinge point, which contains predominantly wedge-shaped cells, and in adjacent lateral areas of the neural plate, which contain predominantly spindle-shaped cells. Both the DNA-synthetic phase and non-DNA synthetic portion of the cell-cycle were significantly longer in the median hinge point than in lateral neuroepithelial areas, some nuclei in both regions were located basally during these phases, and virtually all basal nuclei in the median hinge point were large. Additionally, the mitotic phase was significantly shorter in the median hinge point than in lateral areas. We present a model to explain how alteration of the cell-cycle in the median hinge point could generate wedging of cells in this region.
Similar content being viewed by others
References
Baserga R (1985) The Biology of Cell Reproduction. Harvard University Press, Cambridge, Massachusetts, p 20
Baserga R, Malamud D (1969) Autoradiography. Techniques and Application. Harper & Row, New York, pp 134, 254
Burnside B (1973) Microtubules and microfilaments in amphibian neurulation. Am Zool 13:989–1006
Folkman J, Moscona A (1978) Role of cell shape in growth control. Nature 273:345–349
Fujita S (1962) Kinetics of cellular proliferation. Exp Cell Res 28:52–60
Gordon R (1985) A review of the theories of vertebrate neurulation and their relationship to the mechanics of neural tube birth defects. J Embryol Exp Morphol 89 (Suppl.):229–255
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–92
Johnson HA (1961) Some problems associated with the histological study of cell proliferation kinetics. Cytologia 26:32–41
Karfunkel P (1974) The mechanisms of neural tube formation. Int Rev Cytol 38:245–271
Kopriwa BM, Leblond CP (1962) Improvements in the coating technique of radioautography. J Histochem Cytochem 10:269–284
Langman J, Guerrant RL, Freeman BG (1966) Behavior of neuro-epithelial cells during closure of the neural tube. J Comp Neurol 127:399–412
Lillie RD (1965) Histopathologic Technic and Practical Histochemistry. McGraw-Hill, New York, pp 149–150, 270
Martin A, Langman J (1965) The development of the spinal cord examined by autoradiography. J Embryol Exp Morphol 14:25–35
Sauer FC (1935) Mitosis in the neural tube. J Comp Neurol 62:377–405
Sauer ME, Chittenden AC (1959) Deoxyribonucleic acid content of cell nuclei in the neural tube of the chick embryo: Evidence for intermitotic migration of nuclei. Exp Cell Res 16:1–6
Schoenwolf GC (1982) On the morphogenesis of the early rudiments of the developing central nervous system. Scann Electr Microsc 1982(1):289–308
Schoenwolf GC (1985) Shaping and bending of the avian neuroepithelium: Morphometric analyses. Dev Biol 109:127–139
Schoenwolf GC, Franks MV (1984) Quantitative analyses of changes in cell shapes during bending of the avian neural plate. Dev Biol 105:257–272
Schoenwolf GC, Folsom D, Moe A (1987) A re-examination of the role of microfilaments in neurulation in the chick embryo Anat Rec (in press)
Schroeder TE (1970) Neurulation in Xenopus laevis. An analysis and model based upon light and electron microscopy. J Embryol Exp Morphol 23:427–462
Smith JL, Schoenwolf GC (1987) Cell cycle and neuroepithelial cell shape during bending of the chick neural plate. Anat Rec 218:196–206
Spratt N, Jr (1947) Development in vitro of the early chick blastoderm explanted on yolk and albumen extract saline-agar substrata. J Exp Zool 106:345–365
Steel GG (1968) Cell loss from experimental tumours. Cell Tissue Kinet 1:193–207
Szarski H (1976) Cell size and nuclear DNA content in vertebrates. Int Rev Cytol 44:93–111
Watterson RL (1965) Structure and mitotic behavior of the early neural tube. In: DeHaan RL, Ursprung H (eds) Organogenesis. Holt, Rinehart, and Winston, New York, pp 129–159
Watterson RE, Schoenwolf GC (1984) Laboratory Studies of Chick, Pig, and Frog Embryos. Guide and Atlas of Vertebrate Embryology (5th ed). Burgess, Minneapolis, p 101
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Smith, J.L., Schoenwolf, G.C. Role of cell-cycle in regulating neuroepithelial cell shape during bending of the chick neural plate. Cell Tissue Res. 252, 491–500 (1988). https://doi.org/10.1007/BF00216636
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00216636