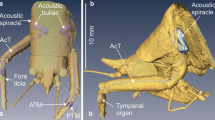
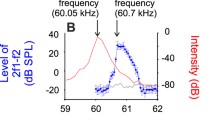
Summary
Acoustic stimuli near 60 kHz elicit pronounced resonance in the cochlea of the mustached bat (Pteronotus parnellii parnellii). The cochlear resonance frequency (CRF) is near the second harmonic, constant frequency (CF2) component of the bat's biosonar signals. Within narrow bands where CF2 and third harmonic (CF3) echoes are maintained, the cochlea has sharp tuning characteristics that are conserved throughout the central auditory system. The purpose of this study was to examine the effects of temperature-related shifts in the CRF on the tuning properties of neurons in the cochlear nucleus and inferior colliculus.
Eighty-two single and multi-unit recordings were characterizedin 6 awake bats with chronically implanted cochlear microphonic electrodes. As the CRF changed with body temperature, the tuning curves of neurons sharply tuned to frequencies near the CF2 and CF3 shifted with the CRF in every case, yielding a change in the unit's best frequency. The results show that cochlear tuning is labile in the mustached bat, and that this lability produces tonotopic shifts in the frequency response of central auditory neurons. Furthermore, results provide evidence of shifts in the frequency-to-place code within the sharply tuned CF2 and CF3 regions of the cochlea. In conjunction with the finding that biosonar emission frequency and the CRF shift concomitantly with temperature and flight, it is concluded that the adjustment of biosonar signals accommodates the shifts in cochlear and neural tuning that occur with active echolocation.
Similar content being viewed by others
Abbreviations
- BF:
-
best frequency
- CF:
-
characteristic frequency
- CF2, CF3:
-
second and third harmonic, constant frequency components of the biosonar signal
- CM:
-
cochlear microphonic
- CN:
-
cochlear nucleus
- CRF:
-
cochlear resonance frequency
- IC:
-
inferior colliculus
- MT:
-
minimum threshold
- OAE:
-
otoacoustic emission
- Q10dB :
-
BF (or CF) divided by the response bandwidth at 10 dB above MT
References
Bell A (1992) Circadian and menstrual rhythms in frequency variations of spontaneous otoacoustic emissions from human ears. Hearing Res 58:91–100
Bonaccorso FJ, Arends A, Genoud M, Cantoni D, Morton T (1992) Thermal ecology of moustached and ghost-faced bats (Mormoopidae) in Venezuela. J Mammal 73:365–378
Brown CR, Bernard RTF (1991) Validation of subcutaneous temperature as a measure of deep body temperature in small bats. J Zool Lond 224:315–346
Cody AR, Russell IJ (1986) The response of mammalian cochlear hair cells to acoustic overstimulation. In: Salvi RJ, Henderson D, Hamernik RP, Colletti V (eds) Basic and applied aspects of noise-induced hearing loss. Plenum, New York, pp 149–160
Covey E, Vater M, Casseday JH (1991) Binaural properties of single units in the superior olivary complex of the mustached bat. J Neurophysiol 66:1080–1094
Cowles RB (1947) Vascular changes in the wings of bats. Science 105:362–363
Dallos P, Harris DM (1978) Properties of auditory nerve responses in the absence of outer hair cells. J Neurophysiol 41:365–383
Davis H (1983) An active process in cochlear mechanics. Hearing Res 9:79–90
De Boer E (1983a) No sharpening? A challenge for cochlear mechanics. J Acoust Soc Am 73:567–573
De Boer E (1983b) On active and passive cochlear models — toward a generalised analysis. J Acoust Soc Am 73:574–576
De Boer E (1983) Power amplification in an active model of the cochlea — short-wave case. J Acoust Soc Am 73:577–579
Eatock RA, Manley GA (1976) Temperature effects on single auditory nerve fibre responses. J Acoust Soc Am Suppl 60:S80
Eatock RA, Manley GA (1981) Auditory nerve fibre activity in the Tokay gecko. II. Temperature effect on tuning. J Comp Physiol 142:219–226
Ehret G, Merzenich MM (1988) Complex sound analysis (frequency resolution, filtering and spectral integration) by single units of the inferior colliculus of the cat. Brain Res Rev 13:139–163
Ehret G, Moffat AJM (1985) Inferior colliculus of the house mouse. II. Single unit responses to tones, noise and tone-noise combinations as a function of sound intensity. J Comp Physiol A 156:619–635
Gummer AW, Klinke R (1983) Influence of temperature on primary-like units in the guinea pig cochlear nucleus. Hearing Res 12:367–380
Haggerty HS (1989) Spontaneous otoacoustic emissions: evidence for circadian rhythm of frequency variation. J Acoust Soc Am Suppl 86:S44
Haggerty HS (1990) Spontaneous otoacoustic emissions: evidence for a monthly rhythm of frequency variation in women. Assoc Res Otolaryngol Abstr 13:233
Harrison JB (1965) Temperature effects on responses in the auditory system of the little brown bat Myotis l. lucifugus. Physiol Zool 38:34–48
Harrison RV, Evans EF (1982) Reverse correlation study of cochlear filtering in normal and pathological guinea pig ears. Hearing Res 6:303–314
Henson MM (1973) Unusual nerve-fiber distribution in the cochlea of the bat, Pteronotus p. parnellii (Gray). J Acoust Soc Am 53:1739–1740
Henson MM (1978) The basilar membrane of the bat, Pteronotus p. parnellii. Am J Anat 153:143–157
Henson MM, Henson OW Jr (1991) Specializations for sharp tuning in the mustached bat: the tectorial membrane and spiral limbus. Hearing Res 56:122–132
Henson MM, Henson OW Jr, Goldman LJ (1977) The perilymphatic spaces in the cochlea of the bat, Pteronotus p. parnellii (Gray). Anat Rec 187:767
Henson MM, Henson OW, Jr, Jenkins DB (1984) The attachment of the spiral ligament to the cochlear wall: anchoring cells and the creation of tension. Hearing Res 16:231–242
Henson OW Jr, Henson MM (1988) Morphometric analysis of cochlear structures in the mustached bat, Pteronotus parnellii parnellii. In: Nachtigall PE, Moore PWB (eds) Animal sonar: Processes and performance. Plenum Press, New York, pp 301–305
Henson OW Jr, Kobler JB (1979) Body temperature and its effect on the CM audiogram of the bat, Pteronotus p. parnellii. Anat Rec 193:744
Henson OW Jr, Pollak GD, Kobler JB, Henson MM, Goldman LJ (1982) Cochlear microphonic potentials elicited by biosonar signals in flying bats, Pteronotus p. parnellii. Hearing Res 7:127–147
Henson OW Jr, Schuller G, Vater M (1985) A comparative study of the physiological properties of the inner ear in Doppler shift compensating bats (Rhinolophus rouxi and Pteronotus parnellii). J Comp Physiol A 157:587–597
Henson OW Jr, Koplas P, Keating AW, Huffman RF, Henson MM (1990) Cochlear resonance in the mustached bat: behavioral adaptations. Hearing Res 50:259–274
Huffman RF, Henson OW Jr (1991) Cochlear and CNS tonotopy: normal physiological shifts in the mustached bat. Hearing Res 56:79–85
Huffman RF, Henson OW Jr (1992) Labile cochlear tuning in the mustached bat. I. Concomitant shifts in biosonar emission frequency. J Comp Physiol A 171:725–734
Khanna SM, Leonard DGB (1982) Basilar membrane tuning in the cat cochlea. Science 215:305–306
Khanna SM, Leonard DGB (1986a) Measurement of basilar membrane vibrations and evaluation of the cochlear condition. Hearing Res 23:37–53
Khanna SM, Leonard DGB (1986b) Relationship between basilar membrane tuning and hair cell condition. Hearing Res 23:55–70
Kim DO, Neely ST, Molnar CE, Matthews JW (1980) An active cochlear model with negative damping in the partition: comparison with Rhode's ante- and post-mortem observations. In: van den Brink G, Bilsen FW (eds) Psychophysical, physiological and behavioural studies in hearing. Delft University Press, Delft, pp 7–14
Kössl M, Vater M (1985a) Evoked acoustic emissions and cochlear microphonics in the mustache bat, Pteronotus parnellii. Hearing Res 19:157–170
Kössl M, Vater M (1985b) The cochlear frequency map of the mustache bat, Pteronotus parnellii. J Comp Physiol A 157:687–697
Kössl M, Vater M (1990a) Tonotopic organization of the cochlear nucleus of the mustache bat, Pteronotus parnellii. J Comp Physiol A 166:695–709
Kössl M, Vater M (1990b) Resonance phenomena in the cochlea of the mustache bat and their contribution to neuronal response characteristics in the cochlear nucleus. J Comp Physiol A 166:711–720
Liberman MC (1984) Single-neuron labeling and chronic cochlear pathology. I. Threshold shift and characteristic-frequency shift. Hearing Res 16:33–41
Liberman MC, Dodds LW (1984) Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hearing Res 16:55–74
Liberman MC, Mulroy MJ (1982) Acute and chronic effects of acoustic trauma: cochlear pathology and auditory nerve pathophysiology. In: Hamernik R, Henderson D, Salvi R (eds) New perspectives on noise-induced hearing loss. Raven, New York, pp 105–135
Liberman MC, Dodds LW, Learson DA (1986) Structure-function correlation in noise-damaged ears. In: Salvi RJ, Henderson D, Hamernik RP, Colletti V (eds) Basic and applied aspects of noise-induced hearing loss. Plenum, New York, pp 163–177
McNab BK (1969) The economics of temperature regulation in neotropical bats. Comp Biochem Physiol 31:227–268
Moffat AJM, Capranica RR (1976) Effects of temperature on the response of the auditory nerve in the American toad (Bufo americanus). J Acoust Soc Am Suppl 60:S80
Moore DR, Semple MN, Addison PD (1983) Some acoustic properties of neurones in the ferret inferior colliculus. Brain Res 269:69–82
Mott JB, Norton SJ, Neely ST, Warr WB (1989) Changes in spontaneous otoacoustic emissions produced by acoustic stimulation of the contralateral ear. Hearing Res 38:229–242
Neely ST, Kim DO (1983) An active cochlear model showing sharp tuning and high sensitivity. Hearing Res 9:123–130
Neely ST, Kim DO (1986) A model for active elements in cochlear biomechanics. J Acoust Soc Am 79:1472–1480
Ohyama K, Sato T, Wada H, Takasaka T (1992) Frequency instability of the spontaneous otoacoustic emissions in the guinea pig. Assoc Res Otolaryngol Abstr 15:150
Olsen JF, Suga N (1991) Combination-sensitive neurons in the medial geniculate body of the mustached bat: encoding of relative velocity information. J Neurophysiol 65:1254–1274
Pollak GD, Bodenhamer RD (1981) Specialized characteristics of single units in inferior colliculus of mustache bat: frequency representation, tuning, and discharge patterns. J Neurophysiol 46:605–620
Pollak GD, Casseday JH (1989) The neural basis of echolocation in bats. Springer Berlin Heidelberg New York, pp 1–143
Pollak G, Henson OW, Jr., Novick A (1972) Cochlear microphonic audiograms in the “pure tone” bat Chilonycteris parnellii parnellii. Science 176:66–68
Pollak G, Henson OW Jr, Johnson R (1979) Multiple specializations in the peripheral auditory system of the CF-FM bat, Pteronotus parnellii. J Comp Physiol 131:255–266
Probst R, Lonsbury-Martin BL, Martin GK (1991) A review of otoacoustic emissions. J Acoust Soc Am 89:2027–2067
Reeder WG, Cowles RB (1951) Aspects of thermoregulation in bats. J Mammal 4:389–403
Robertson D, Johnstone BM (1979) Aberrant tonotopic organization in the inner ear damaged by kanamycin. J Acoust Soc Am 66:366–469
Robertson D, Cody AR, Bredberg G, Johnstone BM (1980) Response properties of spiral ganglion neurons in cochleas damaged by direct mechanical trauma. J Acoust Soc Am 67:1295–1303
Robles L, Ruggero MA, Rich NC (1986a) Basilar membrane mechanics at the base of the chinchilla cochlea. I. Input-output functions, tuning curves and phase responses. J Acoust Soc Am 80:1364–1374
Robles L, Ruggero MA, Rich NC (1986b) Mössbauer measurements of the mechanical response to single-tone and two-tone stimuli at the base of the chinchilla cochlea. In: Allen JB, Hall JL, Hubbard A, Neely ST, Tubis A (eds) Peripheral auditory mechanisms. Springer Berlin, Heidelberg New York, pp 121–128
Ross LS, Pollak GD (1989) Differential ascending projections to aural regions in the 60 kHz contour of the mustache bat's inferior colliculus. J Neurosci 9:2819–2834
Ross LS, Pollak GD, Zook JM (1988) Origin of ascending projections to an isofrequency region of the mustache bat's inferior colliculus. J Comp Neurol 270:488–505
Salvi R, Perry J, Hamernik RP (1982) Relationships between cochlear pathologies and auditory nerve and behavioural responses following acoustic trauma. In: Hamernik R, Henderson D, Salvi R (eds) New perspectives on noise-induced hearing loss. Raven, New York, pp 165–188
Schermuly L, Klinke R (1985) Change of characteristic frequency of pigeon primary auditory afferents with temperature. J Comp Physiol A 156:209–211
Schmiedt RA (1982) Differential effects of kanamycin and impulsenoise exposure on responses of auditory nerve fibers. In: Hamernik R, Henderson D, Salvi R (eds) New perspectives on noiseinduced hearing loss. Raven, New York, pp 153–163
Sellick PM, Patuzzi R, Johnstone BM (1982) Mesurement of basilar membrane motion in the guinea pig using the Mössbauer technique. J Acoust Soc Am 72:131–141
Sellick PM, Patuzzi R, Johnstone BM (1983) Comparison between the tuning properties of inner hair cells and basilar membrane motion. Hearing Res 10:93–100
Smith JD (1972) Systematics of the chiropteran family Mormoopidae. Univ Kansas Mus Nat Hist Misc Publ 56:1–132
Smolders J, Klinke R (1978) Effect of temperature on tuning properties of primary auditory fibres in caiman and cat. Pflügers Arch Suppl 373:R84
Smolders J, Klinke R (1982) Quantitative investigation of temperature effects in primary auditory fibres of Caiman crocodilus. Arch Otorhinolaryngol 234:203–204
Smolders J, Klinke R (1984) Effects of temperature on the properties of primary auditory fibres of the spectacled caiman (Caiman crocodilus). J Comp Physiol A 155:19–30
Stiebler IB, Narins PM (1990) Temperature-dependence of auditory nerve response properties in the frog. Hearing Res 46:63–82
Studier EH, Wilson DE (1970) Thermoregulation in some Neotropical bats. Comp Biochem Physiol 34:251–262
Suga N, Jen PH-S (1976) Disproportionate tonotopic representation for processing CF-FM sonar signals in the mustache bat auditory cortex. Science 194:542–544
Suga N, Jen PH-S (1977) Further studies on the peripheral auditory system of the CF-FM bats specialized for fine frequency analysis of Doppler-shifted echoes. J Exp Biol 169:207–232
Suga N, Simmons JA, Jen PH-S (1975) Peripheral specializations for fine analysis of Doppler-shifted echoes in the auditory system of the “CF-FM” bat Pteronotus parnellii. J Exp Biol 63:161–192
Suga N, Niwa H, Taniguchi I, Margoliash D (1987) The personalized auditory cortex of the mustached bat: adaptation for echolocation. J Neurophysiol 58:643–654
Thomas SP (1987) The physiology of bat flight. In: Fenton MB, Racey P, Rayner JMV (eds) Recent advances in the study of bats. Cambridge University Press, Cambridge, pp 75–99
Thomas SP, Suthers RA (1972) The physiology and energetics of bat flight. J Exp Biol 57:317–335
Vater M (1987) Narrow-band frequency analysis in bats. In: Fenton MB, Racey P, Rayner JMV (eds) Recent advances in the study of bats. Cambridge University Press, Cambridge, pp 200–225
Vater M (1988) Cochlear physiology and anatomy in bats. In: Nachtigall PE, Moore PWB (eds) Animal sonar: Processes and performance. Plenum Press, New York, pp 225–241
Zook JM, Leake PA (1989) Connections and frequency representation in the auditory brainstem of the mustache bat, Pteronotus parnellii. J Comp Neurol 290:243–261
Zook JM, Winer JA, Pollak GD, Bodenhamer RD (1985) Topology of the central nucleus of the mustache bat's inferior colliculus: correlation of single unit properties and neuronal architecture. J Comp Neurol 231:530–546
Zwislocki JJ (1980) Theory of cochlear mechanics. Hearing Res 2:171–182
Zwislocki JJ, Kletsky EJ (1980) Micromechanics in the theory of cochlear mechanics. Hearing Res 2:505–512
Zwislocki JJ, Kletsky EJ (1982) What basilar-membrane tuning says about cochlear micromechanics. Am J Otolaryngol 3:48–52
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Huffman, R.F., Henson, O.W. Labile cochlear tuning in the mustached bat. J Comp Physiol A 171, 735–748 (1993). https://doi.org/10.1007/BF00213070
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00213070