Abstract
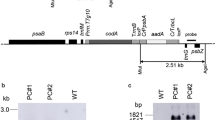
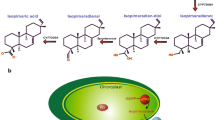
Certain higher plants synthesize and accumulate glycine betaine, a compound with osmoprotectant properties. Biosynthesis of glycine betaine proceeds via the pathway choline → betaine aldehyde → glycine betaine. Plants such as tobacco (Nicotiana tabacum L.) which do not accumulate glycine betaine lack the enzymes catalyzing both reactions. As a step towards engineering glycine betaine accumulation into a non-accumulator, spinach and sugar beet complementary-DNA sequences encoding the second enzyme of glycine-betaine synthesis (betaine aldehyde dehydrogenase, BADH, EC 1.2.1.8) were expressed in tobacco. Despite the absence of a typical transit peptide, BADH was targeted to the chloroplast in leaves of transgenic plants. Levels of extractable BADH were comparable to those in spinach and sugar beet, and the molecular weight, isoenzyme profile and K m for betaine aldehyde of the BADH enzymes from transgenic plants were the same as for native spinach or sugar beet BADH. Transgenic plants converted supplied betaine aldehyde to glycine betaine at high rates, demonstrating that they were able to transport betaine aldehyde across both the plasma membrane and the chloroplast envelope. The glycine betaine produced in this way was not further metabolized and reached concentrations similar to those in plants which accumulate glycine betaine naturally. Betaine aldehyde was toxic to non-transformed tobacco tissues whereas transgenic tissues were resistant due to detoxification of betaine aldehyde to glycine betaine. Betaine aldehyded ehydrogenase is therefore of interest as a potential selectable marker, as well as in the metabolic engineering of osmoprotectant biosynthesis.
Similar content being viewed by others
Abbreviations
- BADH:
-
betaine aldehyde dehydrogenase
- bp:
-
base pairs
- FAB-MS:
-
fast atom bombardment-mass spectrometry
- GAPDH:
-
NADP-linked glyceraldehyde-3-phosphate dehydrogenase
References
An, G., Ebert, P.R., Mitra, A., Ha, S.B. (1988) Binary vectors. In: Plant molecular biology manual A3, pp. 1–19, Gelvin, S.B., Schilperoort, R.A., eds. Kluwer, Dordrecht
Arakawa, K., Takabe, T., Sugiyama, T., Akazawa, T. (1987) Purification of betaine-aldehyde dehydrogenase from spinach leaves and preparation of its antibody. J. Biochem. 101, 1485–1488
Arnon, D.I. (1949) Copper enzymes in chloroplasts. Polyphenoloxidases in Beta vulgaris. Plant Physiol. 24, 1–15
Berry-Lowe, S.L., Schmidt, G.W. (1991) Chloroplast protein transport. In: Cell culture and somatic cell genetics of plants, vol. 7A: The molecular biology of plastids, pp. 257–302, Bogorad, L., Vasil, I.K., eds. Academic Press, New York
Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254
Brouquisse, R., Weigel, P., Rhodes, D., Yocum, C.F., Hanson, A.D. (1989) Evidence for a ferredoxin-dependent choline monooxygenase from spinach chloroplast stroma. Plant Physiol. 90, 322–329
Budar, F., Thia-Toong, L., Van Montagu, M., Hernalsteens, J.P. (1986) Agrobacterium-mediated gene transfer results mainly in transgenic plants transmitting T-DNA as a single Mendelian factor. Genetics 114, 303–313
Byerrum, R.U., Sato, C.S., Ball, C.D. (1956) Utilization of betaine as a methyl group donor in tobacco. Plant Physiol. 31, 374–377
Cromwell, B.T. (1955) The tobacco alkaloids. In: Modern methods of plant analysis, vol. 4, pp. 487–516, Paech, K., Tracey, M.V. eds. Springer, Berlin Heidelberg New York
Csonka, L.N., Hanson, A.D. (1991) Prokaryotic osmoregulation: genetics and physiology. Annu. Rev. Microbiol. 45, 569–606
Flavell, R.B., Dart, E., Fuchs, R.L., Fraley, R.T. (1992) Selectable marker genes: safe for plants? Bio/Technology 10, 141–144
Grimm, B., Ish-Shalom, D., Even, D., Glaczinski, H., Ottersbach, P., Ohad, L., Kloppstech, K. (1989) The nuclear-coded chloroplast 22-kDa heat-shock protein of Clamydomonas: evidence for translocation into the organelle without a processing step. Eur. J. Biochem. 182, 539–546
Hanson, A.D., Wyse, R. (1982) Biosynthesis, translocation, and accumulation of betaine in sugar beet and its progenitors in relation to salinity. Plant Physiol. 70, 1191–1198
Hedrick, J.L., Smith, A.J. (1968) Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch. Biochem. Biophys. 126, 155–164
Kao, K.N., Michayluk, M.R. (1975) Nutritional requirements for growth of Vicia hajastana cells and protoplasts at a very low population density in liquid media. Planta 126, 105–110
Keegstra, K., Olsen, L.J., Theg, S.M. (1989) Chloroplastic precursors and their transport across the envelope membranes. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 471–501
Ladyman, J.A.R., Hitz, W.D., Hanson, A.D. (1980) Translocation and metabolism of glycine betaine. Planta 150, 191–196
LeRudulier, D., Strom, A.R., Dandekar, A.M., Smith, L.T., Valentine, R.C. (1984) Molecular biology of osmoregulation. Science 224, 1064–1068
Lilley, R.M.., Fitzgerald, M.P., Rienits, K.G., Walker, D.A. (1975) Criteria of intactness and the photosynthetic activity of spinach chloroplast preparations. New Phytol. 75, 1–10
Luck, H. (1965) Catalase. In: Methods of enzymatic analysis, pp. 886–888, Bergmeyer, H.U. ed. Academic Press, New York
McCue, K.F., Hanson, A.D. (1990) Drought and salt tolerance: towards understanding and application. Trends Biotechnol. 8, 358–362
McCue, K.F., Hanson, A.D. (1992) Salt-inducible betaine aldehyde dehydrogenase from sugar beet: cDNA cloning and expression. Plant Mol. Biol. 18, 1–11
Murashige, T., Skoog, F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473–497
Puissant, C., Houdebine, L.-M. (1990) An improvement of the single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. BioTechniques 8, 148–149
Racker, E. (1950) Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim. Biophys. Acta 4, 211–214
Rathinasabapathi, B., King, J. (1992) An ultrastructural investigation on the polyethylene glycol-induced adhesion of tobacco nuclei and uptake of micronuclei by soybean protoplasts. Plant Cell Tissue Organ Culture 29, 207–214
Rhodes, D., Hanson, A.D. (1993) Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44, 357–384
Rhodes, D., Rich, P.J., Myers, A.C., Reuter, C.C., Jamieson, G.C. (1987) Determination of betaines by fast atom bombardment mass spectrometry: identification of glycine betaine deficient genotypes of Zea mays. Plant Physiol. 84, 781–788
Sambrook, J., Fritsch, E.F., Maniatis, T. (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Press, New York
Somero, G.N. (1986) Protons, osmolytes, and fitness of internal milieu for protein function. Am. J. Physiol. 251, R197-R213
Tarczynski, M.C., Jensen, R.G., Bohnert, H.J. (1993) Stress protection of transgenic tobacco by production of the osmolyte mannitol. Science 259, 508–510
Weigel, P., Weretilnyk, E.A., Hanson, A.D. (1986) Betaine aldehyde oxidation by spinach chloroplasts. Plant Physiol. 82, 753–759
Weretilnyk, E.A., Hanson, A.D. (1988) Betaine aldehyde dehydrogenase polymorphism in spinach: genetic and biochemical characterization. Biochem. Genet. 26, 143–151
Weretilnyk, E.A., Hanson, A.D. (1989) Betaine aldehyde dehydrogenase from spinach leaves: purification, in vitro translation of the mRNA, and regulation by salinity. Arch. Biochem. Biophys. 271, 56–63
Weretilnyk, E.A., Hanson, A.D. (1990) Molecular cloning of a plant betaine-aldehyde dehydrogenase, an enzyme implicated in adaptation to salinity and drought. Proc. Natl. Acad. Sci. USA 87, 2745–2749
Weretilnyk, E.A., Bednarek, S., McCue, K.F., Rhodes, D., Hanson, A.D. (1989) Comparative biochemical and immunological studies of the glycine betaine synthesis pathway in diverse families of dicotyledons. Planta 178, 342–352
Wolosiuk, R.A., Buchanan, B.B. (1976) Studies on the regulation of chloroplast NADP-linked glyceraldehyde-3-phosphate dehydrogenase. J. Biol. Chem. 251, 6456–6461
Wyn Jones, R.G. (1984) Phytochemical aspects of osmotic adaptation. Recent Adv. Phytochem. 18, 55–78
Wyn Jones, G.R., Rippin, A.J., Storey, R. (1973) Metabolism of choline in the rhizosphere and its possible influence on plant growth. Pestic. Sci. 4, 375–383
Author information
Authors and Affiliations
Additional information
We thank Dr. G. An for the gift of the vector pGA643 and Mr. Sylvain Lebeurier for help in maintaining plants. This work was supported, in part, by grants from the Natural Sciences and Engineering Research Council of Canada, the Rockefeller Foundation, and the U.S. Department of Agriculture, and by gifts from CIBAGEIGY Biotechnology.
Rights and permissions
About this article
Cite this article
Rathinasabapathi, B., McCue, K.F., Gage, D.A. et al. Metabolic engineering of glycine betaine synthesis: plant betaine aldehyde dehydrogenases lacking typical transit peptides are targeted to tobacco chloroplasts where they confer betaine aldehyde resistance. Planta 193, 155–162 (1994). https://doi.org/10.1007/BF00192524
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00192524