Abstract
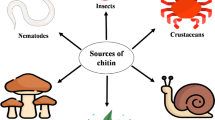
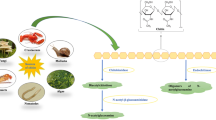
Chitin is produced in enormous quantities in the biosphere, chiefly as the major structural component of most fungi and invertebrates. Its degradation is chiefly by bacteria and fungi, by chitinolysis via chitinases, but also via deacetylation to chitosan, which is hydrolysed by chitosanases. Chitinases and chitosanases have a range of roles in the organisms producing them: autolytic, morphogenetic or nutritional. There are increasing examples of their roles in pathogenesis and symbiosis. A range of chitinase genes have been cloned, and the potential use for genetically manipulated organisms over-producing chitinases is being investigated. Chitinases also have a range of uses in processing chitinous material and producing defined oligosaccharides.
Similar content being viewed by others
References
Aruchami, M, Sundara-Rajulu, G & Gowri, N (1986) Distribution of deacetylase in arthropoda. In: Muzzarelli, R, Jeuniaux, C & Gooday, GW (Eds) Chitin in Nature and Technology (pp 263–265). Plenum Press, New York
Baross, JA, Tester, PA & Morita, RY (1978) Incidence, microscopy and etiology of exoskeleton lesions in the tanner crab Chionectes tanner. J Fish Res Board Can 35: 1141–1149
Barrett-Bee, K & Hamilton, M (1984) The detection and analysis of chitinase activity from the yeast form of Candida albicans. J Gen Microbiol 130: 1857–1861
Bartnicki-Garcia, S (1973) Fundamental aspects of hyphal morphogenesis. In: Ashworth, JM & Smith, JE (Eds) Microbial Differentiation (pp 245–268). Cambridge University Press, Cambridge
Bartnicki-Garcia, S & Lippman, E (1982) Fungal wall composition. In Laskin, AJ & Lechevalier, HA (Eds) CRC Handbook of Microbiology, 2nd Ed. Vol. IV, Microbial Composition: Carbohydrates, Lipids and Minerals (pp 229–252). CRC Press, Boca Raton
Bassler, B., Gibbons, P & Roseman, S (1989) Chemotaxis to chitin oligosaccharides by Vibrio furnissii, a chitinivorous marine bacterium. Biochem Biophys Res Comm 161: 1172–1176
Benhamou, N & Asselin, A (1989) Attempted localization of a substrate for chitinases in plant cells reveals abundant N-acetyl-D-glucosamine residue in secondary walls. Biol Cell 67: 341–250
Berkeley, RCW (1979) Chitin, chitosan and their degradative enzymes. In: Berkeley, RCW, Gooday, GW & Ellwood, DC (Eds) Microbial Polysaccharides and Polysaccharases (pp 205–236). Academic Press, London
Beyer, M & Diekmann, H (1985) The chitinase system in Streptomyces sp. ATCC 11238 and its significance for fungal cell wall degradation. Appl Microbiol Biotech 23: 14–146
Bibochka, MJ & Khachatourians (1988) N-Acetyl-D glucosamine-mediated regulation of extracellular protease in the entomopathogenic fungus Beauvaris bassiana. Appl Environ Microbiol 54: 2699–2704
Blackwell, J (1988) Physical methods for the determination of chitin structure and conformation. Meth Enzymol 161: 435–442
Borkott, H & Insam, H (1990) Symbiosis with bacteria enhances the use of chitin by the springtail, Folsomia candida (Collembola). Biol Fert Soils 9: 126–129
Bradbury, P, Deroux, G & Campillo, A (1987) The feeding of a chitinivorous ciliate. Tissue Cell 19: 351–363
Broglie, KE, Gaynor, JJ Broglie, RM (1986) Ethylene-regulated gene expression: molecular cloning of the genes encoding an endochitinase from Phaseolus vulgaris. Proc Nat Acad Sci USA 86: 6820–6824
Cabib, E, Silverman, SJ, Sburlati, A & Slater, ML (1990) Chitin synthesis in yeast Saccharomyces cerevisiae. In: Kuhn, PJ, Trinci, APJ, Jung, MJ, Goosey, MW & Copping, LG (Eds) Biochemistry of Cell Walls and Membranes in Fungi (pp 31–41). Springer-Verlag, Berlin
Chan J G (1970) The Occurrence, Taxonomy and Activity of Chitinolytic Bacteria from Sediment, Water and Fauna of Puget Sound. Ph.D. thesis, University of Washington, Seattle
Dackman, C, Chet, I & Nordbring-Hertz, B (1989) Fungal parasitism of the cyst nematode Heterodera schachtii: infection and enzymatic activity. FEMS Microbiol Ecol 62 201–208
Daoust, RA & Gunner, HB (1979) Microbial synergists pathogenic to Lymantria dispar: chitinolytic and fermentative bacterial interactions. J Invert Path 33: 368–377
Datema, R, Ende, Hvan den & Wessels, JGH (1977) The hyphal wall of Mucor mucedo 2. Hexosamine-containing polymers. Eur J Biochem. 80: 621–626
Davis, B & Eveleigh, DE (1984) Chitosanases: occurrence, production and immobilization. In: Zikakis, JP (Ed) Chitin, Chitosan and Related Enzymes (pp 161–179). Academic Press, Orlando
Davis, LL & Bartnicki-Garcia, S (1984) The co-ordination of chitosan and chitin synthesis in Mucor rouxii. J Gen Microbiol 130: 2095–2102
Derksen, ACG & Granados, RR (1988) Alteration of a lepidoteran peritrophic membrane by baculoviruses and enhancement of viral infectivity. Virology 167: 242–250
Dunsmuir, P & Suslow, T (1989) Structure and regulation of organ- and tissue-specific-genes: chitinase genes in plants. In: Schell, J & Vaszil, IK (Eds) Cell Culture and Somatic Cell Genetics in Plants, Vol 6, Molecular Biology of Plant Nuclear Genes (pp 215–227). Academic Press, San Diego
Elango, N, Correa, JV, Cabib, E (1982) Secretory nature of yeast chitinase. J Biol Chem 257: 1398–1400
Flyg, C & Boman, HG (1988) Drosphila genes cut and miniature are associated with the susceptibility to infection in Serratia marcescens. Genet Res 52: 51–56
Fuchs, R, McPherson, S & Drahos, D (1986) Cloning of a Serratia marcescens gene encoding chitinase. Appl Environ Microbiol 51: 504–509
Gooday, GW (1979) A survey of polysaccharase production: a search for phylogenetic implications. In: Berkeley, RCW, Gooday, GW & Ellwood, DC (Eds) Microbial Polysaccharides and Polysaccharaes (pp 437–460). Academic Press, London
(1990a) The ecology of chitin degradation. In: Marshall, KC (Ed) Advances in Microbial Ecology, Vol. 11 (pp 387–430). Plenum Press, New York
(1990b) Inhibition of chitin metabolism. In: Kuhn, PJ, Trinci, APJ, Jung, MJ, Goosey, MW & Copping, LG (Eds) Biochemistry of Cell Walls and Membranes in Fungi (pp 61–79). Springer-Verlag, Berlin
(1990c) Chitinases. In: Leatham, G & Himmel, M (Eds) Enzymes in Biomass Conversion. American Chemical Society Books, Washington
(1990d) Chitin metabolism: a target for antifungal and antiparasitic drugs. Pharmacol Ther Suppl 175–185
Gooday, GW & Gow, NAR (1991) The enzymology of tip growth in fungi. In: Heath, IB (Ed) Tip Growth of Plant and Fungal Cells (pp 31–58). Academic Press, New York
Gooday, GW, Humphreys, AM & McIntosh, WH (1986) Roles of chitinase in fungal growth. In: Muzzarelli, RAA, Jeuniaux, C & Gooday, GW (Eds) (pp 83–91). Plenum Press, New York
Gooday GW, Prosser JI, Hillman K & Cross M (1991) Mineralization of chitin in an estuarine sediment: the importance of the chitosan pathway. Biochem Systematics Ecol (in press)
Gow, NAR & Gooday, GW (1983) Ultrastructure of chitin in hyphae of Candida albicans and other dimorphic and mycelial fungi. Protoplasma 115: 52–58
Hara, S, Yamamura, Y, Fujii, Y, Mega, T & Ikenaka, T (1989) Purification and characterization of chitinase produced by Streptomyces erythraeus. J Biochem 105: 484–489
Hedges, A & Wolfe, RS (1974) Extracellular enzyme from Myxobacter AL-1 that exhibits both β-1,4-glucanase and chitosanase activities. J Bacteriol 120: 844–853
Hellebust, JA & Lewin, J (1977) Heterotrophic nutrition. In: Werner, D (Ed) The Biology of Diatoms (pp 169–197). Blackwell, Oxford
Helmke, E & Weyland, H (1986) Effect of hydrostatic pressure and temperature on the activity and synthesis of chitinases of Antarctic Ocean bacteria. Mar Biol 91: 1–7
Herwig, RP, Staley, JT, Nerini, MK & Braham, HW (1984) Baleen whales: preliminary evidence for forestomach microbial fermentation. Appl Environ Microbiol 47 421–423
Hillman, K, Gooday, GW & Prosser, JI (1989a) The mineralization of chitin in the sediments of the Ythan Estuary, Aberdeenshire, Scotland. Estuarine Coastal Shelf Sci 29: 601–612
(1989b) A simple model system for small scale in vitro study of estuarine sediment ecosystems. Lett Appl Microbiol 4: 41–44
Hirano, S & Tokura, S (1982) Chitin and Chitosan. Japanese Society of Chitin and Chitosan. Tottori
Hood, MA & Meyers, SP (1977) Microbial and chitinoclastic activities associated with Panaeus setiferus. J Oceangraph Soc Japan 33 235–241
Horwitz, M, Reid, J & Ogaydziak, D (1984) Genetic improvements of chitinase production of Serratia marcescens. In: Zikakis, J (Ed) Chitin, Chitosan and Related Enzymes (pp 191–189). Academic Press, Orlando
Humphreys, AM & Gooday, GW (1984a) Properties of chitinase activities from Mucor mucedo: Evidence for a membrane-bound zymogenic form. J Gen Microbiol 130: 1359–1366
(1984b) Phospholipid requirement of microsomal chitinase from Mucor mucedo. Curr Microbiol 11: 187–190
(1984c) Chitinase activities from Mucor mucedo. In: Nombela, C. (Ed) Microbial Cell Wall Synthesis and Autolysis (pp 269–273). Elsevier, Amsterdam
Isaac, S & Gokhale, AV (1982) Autolysis: a tool for protoplast production from Aspergillus nidulans. Trans Br Mycol Soc 78: 389–394
Isogai, A, Sato, M, Sakuda, S, Nakuyama, A (1989) Structure of demethylallosamidin as an insect chitinase inhibitor. Agric Biol Chem 53: 2825–2826
Iten, W & Matile, P (1970) Role of chitinase and other lysosomal enzymes of Coprinus lagopus in the autolysis of fruiting bodies. J Gen Microbiol 61: 301–309
Iverson, KL, Bromel, MC, Anderson, Aw & Freeman, TP (1984) Bacterial symbionts in the sugar beet root maggot Tetanops myopaeformis (von Röder), Appl Environ Microbiol 47: 22–27
Janszen, FH & Wessels, JGH (1970) Enzymic dissolution of hyphal septa in a Basidiomycete. Antonie van Leewenhoek 36: 255–257
Jeuniaux, C (1963) Chitine and Chitinolyse. Masson, Paris
(1982) La chitine dans le régne animal, Bull Soc Zool France 107: 363–386
Jones, J, Grady, K, Suslow, T & Bedbrook, J (1986) Isolation and characterization of genes encoding two distinct chitinase enzymes from Serratia marcescens EMBO J 5: 467–473
Jones, JDG, Dean, C, Gidoni, D, Gilbert, D, Bond-Nutter, D, Nedbrook, J & Dunsmuir, P (1988) Expression of a bacterial chitinase protein in tobacco leaves using two photosynthetic gene promoters. Mol Gen Genet 212: 536–542
Kamei, K, Yamamura, Y, Hara, S & Ikenda, T (1989) Amino acid sequence of chitinase from Streptomyces erythaeus. J Biochem 105: 979–985
Kaneko, T & Colwell, RR (1978) The annual cycle of Vibrio parahaemolyticus in Chesapeake Bay. Microbial Ecol 4: 135–155
Kohlmeyer, J (1972) Marine fungi deteroriating chitin of hydrozoa and keratin-like annelid tubes. Mar Biol 12: 277–284
Kole, MM & Altosaar, I (1985) Increased chitinase production by a non-pigmented mutant of Serratia marcescens. FEMS Microbial Lett 26: 265–269
Kuhl, J, Nittinger, J & Siebert, G (1978) Vervetang von Krillschalen in Futterungsversuchen an de Ratte, Arch Fischereiwiss 29: 99–103
Kuranda, MJ & Robbins, PW (1988) Cloning and heterologus expression of glycosidase genes from Saccharomyces cerevisiae. Proc Nat Acad Sci USA 84: 2585–2589
Lindsay, GJH & Gooday, GW (1985a) Action of chitinase in spines of the diatom Thalassiosira fluviatilis. Carbohydr Polymers 5 131–140
(1985b) Chitinolytic enzymes and the bacterial microflora in the digestive tract of cod, Gadus morhua. J Fish Biol 26: 255–265
Lindsay, GJH, Walton, MJ, Adron, JW, Fletcher, TC, Cho, CY & Cowey, CB (1984) The growth of rainbow trout (Salmo gairdneri) given diets containing chitin and its relationships to chitinolytic enzymes and chitin digestibility. Aquaculture 37: 315–334
Linthorst HJM, Loon LC van, Rossum CMA van, Mayer, A, Bol JF, Roekel JSC van, Meulenhoff EJS & Cornelissen BJC (1990) Analysis of acid and basic chitinases from tobacco and petunia and their constitutive expression in transgenic tobacco. Mol Plant-Microbe Interact (in press)
Lund, P, Lee, RY & Dunsmuir, P (1989) Bacterial chitinase is modified and secreted in transgenic tobacco. Plant Physiol 91: 130–135
Lynch, J (1987) In vitro identification of Trichoderma harzianum as a potential antagonist of plant pathogens. Curr Microbiol 16: 49–53
Lysenko, O (1976) Chitinase of Serratia marcescens and its toxicity to insects, J Invertebr Pathol 27: 385–386
Mauch, F, Mauch-Mani, B & Boller, T (1988) Antifungal hydrolases in pea tissue. Inhibition of fungal growth by combination of chitinase and β-1,3-glucanase. Plant Physiol 87: 936–942
Mauch, F & Staehelin, LA (1989) Functional implications of the subcellular localization of ethylene-induced chitinase and β-1,3-glucanase in bean leaves. Plant Cell 1: 447–457
Maudlin, I & Welburn, SC (1988) Parasit Today 4: 109–111
Monaghan RL (1975) The Discovery, Distribution and Utilization of Chitosanase. Ph.D. Thesis, Rutgers University, New Brunswick, N.J.
Monaghan, RL, Eveleigh, DE, Tewari, RP & Reese, ET (1973) Chitosanase, a novel enzyme, Nature (London) New Biol 245: 78–80
Monreal, J & Reese, ET (1969) The chitinase of Serratia marcescens. Can J Microbiol 15: 689–696
Murray, CL & Lovett, J S (1966) Nutritional requirements of the chytrid, Karlingia asterocysta, an obligate chitinophile. Am J Bot 53: 469–476
Muzzarelli RAA & Pariser ER (1978) Proceedings of the First International Conference on Chitin/Chitosan. MIT Sea Grant Report MITSG
Muzzarelli, RAA, Jeuniaux, C & Gooday, GW (1986) Chitin in Nature and Technology. Plenum, New York
Nanjo, F, Sakai, K, Ishikawa, M, Isobe, K & Usui, T (1989) Agric Biol Chem 53: 2189–2195
Orunsi, NA & Trinci, APJ (1985) Growth of bacteria on chitin, fungal cell walls and fungal biomass, and the effect of extracellular enzymes produced by these cultures on the antifungal activity of amphotericin B. Microbios 43: 17–30
Patton, RS & Chandler, PT (1975) In vivo digestibility evaluation of chitinous material. J Dairy Sci 58: 397–403
Payne, S, Ahl, P, Moyer, M, Harper, A, Beck, J, Meins, F & Ryals, J (1990) Isolation of complementary DNA clones encoding pathogeneis-related proteins P and Q, two acid chitinases from tobacco. Proc Nat Acad Sci USA 87: 98–102
Peberdy, J (1983) Genetic recombination in fungi following protoplast fusion and transformation. In: Smith, JE (Ed) Fungal Differentiation (pp 559–581). Dekker, New York
Pel, R & Gottschal, JC (1986a) Chitinolytic communities from an anaerobic estuarine environment, In Muzzarelli, RAA, Jeuniaux, C & Gooday, GW (Eds) Chitin in Nature and Technology (pp 539–546). Plenum, New York
(1986b) Stimulation of anaerobic chitin degradation in mixed cultures, Antonie van Leewenhoek 52: 359–360
(1989) Interspecies interaction based on transfer of a thioredoxin-like compound in anaerobic chitin-degrading mixed cultures. FEMS Microbiol Ecol 62: 349–358
Pel, R, Wessels, G, Aalfs, H & Gottschal, JC (1989) Chitin degradation by Clostridium sp. strain 9.1 in mixed cultures with saccharolytic and sulphate-reducing bacteria. FEMS Microbiol Ecol 62: 191–200
Pel, R, Wijngaard, AjVan Den, Epping, E & Gottschal, JC (1990) Comparison of the chitinolytic properties of Clostridium sp. strain 9.1 and a chitin degrading bacterium from the intestinal tract of the plaice Pleuronectes platessa (L.). J Gen Microbiol 136: 695–704
Pelletier, A & Sygusch, J (1990) Purification and characterization of three chitosanase activities from Bacillus megaterium P1. Appl Environ Microbiol 56: 844–848
Pelletier, A, Lemire, I, Sygusch, J, Chornet, E & Overend, RP (1990) Chitin/chitosan transformation by thermo-mechano-chemical treatment including characterization by enzymatic depolymerisation. Biotech Bioeng 36: 310–315
Peter, MG, Kegel, G & Keller, R (1986) Structural studies on sclerotized insect cuticle. In: Muzzarelli, R, Jeuniaux, C, Gooday, GW (Eds) Chitin in Nature and Technology (pp 21–28). Plenum Press, New York
Petz, W, Foissner, W, Wirnsberger, E, Kruatgartner, Wd & Adam, H (1986) Mycophagy, a new feeding strategy in autochthonous ciliates. Naturwissenschaften 73: S.560–561
Pope, AMS & Davies, DAL (1979) The influence of carbohydrases on the growth of fungal pathogens in vitro and in vivo. Postgrad Med J 55: 674–676
Poulicek, M, Voss-Foucart, MF & Jeuniaux, C (1986) Chitinoproteic complexes and mineralization in mollusk skeletal structures. In: Muzzarelli, R, Jeuniaux, C, Gooday, GW (Eds) Chitin in Nature and Technology (pp 7–12). Plenum Press, New York
Reid, JD & Ogrydziak, DM (1981) Chitinase-overproducing mutant of Serratia marcescens. Appl Environ Microbiol 41: 664–669
Reichardt, W, Gunn, B and Colwell, RR (1983) Ecology and taxonomy of chitinoclastic Cytophaga and related chitin-degrading bacteria isolated from an estuary, Microb Ecol 9: 273–294
Reisert, PS & Fuller, MS (1962) Decomposition of chitin by Chytridiomyces species. Mycologia 54: 647–657
Reyes, F, Perez-Leblic, MI, Martinez, MJ & Lahoz, R (1984) Protoplast production from filamentous fungi with their own autolytic enzymes. FEMS Microbiol Lett 24: 281–283
Reyes, F, Lahoz, R, Martinez, MJ & Alfonso, C (1985) Chitosanases in the autolysis of Mucor rouxii. Mycopathologia 89: 181–187
Robbins, PW, Albright, C & Benfield, B (1988) Cloning and expression of a Streptomyces plicatus chitinase (chitinase-63) in Escherichia coli. J Biol Chem 263: 443–447
Roberts, RL & Cabib, E (1982) Serratia marcescens chitinase: one-step purification and use for the determination of chitin. Anal Biochem 127: 402–412
Roberts, WK & Selitrennikoff, CP (1988) Plant and bacterial chitinases differ in antifungal activity. J Gen Microbiol 134: 169–176
Sakuda, S, Isogai, A, Matsumoto, S & Suzuki, A (1987a) Search for microbial insect growth regulators II. Allosamidin, a novel insect chitinase inhibitor. J Antibiot 40: 296–300
Sakuda, S, Isogai, A, Makita, T, Matsumoto, S, Koseki, K, Kodama, H & Suzuki, A (1987b) Structures of Allosamidins, novel insect chitinase inhibitors, produced by actinomycetes. Agric Biol Chem 51: 3251–3259
Sakuda, A, Nishimoto, Y, Ohi, M, Watanabe, M, Takayama, T, Isogai, A & Yamada, Y (1990) Effects of demethylallosamidin, a potent yeast chitinase inhibitor, on the cell division of yeast. Agric Biol Chem 54: 1333–1335
Sassen, MMA (1965) Breakdown of the plant cell wall during the cell fusion process. Acta Bot Neerlandica 14: 165–196
Seki, H & Taga, N (1965) Microbial studies on the decomposition of chitin in marine environment-VI. Chitinoclastic bacteria in the digestive tract of whales from the Antarctic Ocean. J Oceanogr. Soc Japan 20: 272–277
Shapira, R, Ordentlich, A, Chet, I & Oppenheim, AB (1989) Control of plant diseases by chitinase expressed from cloned DNA in Escherichia coli. Phytopathology 79: 1246–1249
Shinshi, H, Beuhaus, J, Ryals, J & Meins, F (1990) Structure of a tobacco chitinase gene: evidence that different chitinase genes can arise by a transposition of sequences encoding a cysteine-rich domain. Plant Mol Biol 14: 357–368
Sietsma, JH, Vermeulen, CA & Wessels, JGH (1986) The role of chitin in hyphal miorphogenesis. In Muzzarelli, R, Jeuniaux, C & Gooday, GW (Eds) Chitin in Nature and Technology (pp 6369). Plenum Press, New York
Sivan, A & Chet, I (1989) Degradation of fungal cell walls by lytic enzymes of Trichoderma harzianum. J Gen Microbiol 135: 675–682
Skjak-Braek, G, Anthosen, T & Sandford, P (1989) Chitin and Chitosan, Elsevier, London
Smith, RJ & Grula, EA (1983) Chitinase is an inducible enzyme in Beauvaria bassiana. I Invertebr Path 42 319–326
Bneh, B (1981) The use of rhizophere chitinolytic bacteria for biological control. Phytopath Zeitscrift 100: 251–256
Soderhall, K & Unestam, T (1975) Properties of extracellular enzymes from Aphanomyces astaci and their relevance in the penetration process of crayfish cuticle. Physiol Plant 35: 140–146
Somers, PJB, Yao, RC, Doolin, LR, McGowan, MJ, Fakuda, DS & Mynderse, JS (1987) Method for the detection and quantitation of chitinase inhibitors in fermentation broths: Isolation and insect life cycle. Effect of A82516. J Antibiot 40: 1751–1756
Soto-Gil, RW & Zyskind, JW (1984) Cloning of Vibrio harveyi chitinase and chitobiase gene in Escherichia coli. In: Zikakis, JP (Ed) Chitin, Chitosan and Related Enzymes (pp 209–223). Academic Press, Orlando
Srivastava, AK, Defago, G & Boller, T (1985) Secretion of chitinase by Aphanocladium album, a hyperparsite of wheat. Experientia 41: 1612–1613
St Leger, RJ, Cooper, RM & Charnley, AK (1986) Cuticle-degrading enzymes of entomopathogenic fungi: regulation of production of chitinolytic enzymes. J Gen Microbiol 132: 1509–1517
Surarit, R, Gopel, PK & Shepherd, MG (1988) Evidence for a glycosidic linkage between chitin and glucan in the cell wall of Candida albicans. J Gen Microbiol 134: 1723–1730
Suslow TV & Jones J (1988) Chitinase-producing bacteria. US Patent No 4751081
Suzuki, K, Mikami, T, Okawa, Y, Tokora, A, Suzuki, S & Suzuki, M (1986) Antitumour effect of hexa-N-acetylchitohexase and chitohexaose. Carb Res 151: 403–408
Takiguchi, Y & Shimahara, K (1988) N,N-Diacetylchitobiose production from chitin by Vibrio anguillarum strain E-383a. Lett Appl Microbiol 6 129–131
(1989) Isolation and identification of a thermophilic bacterium producing N,N-diacetylchitobiose from chitin. Agric Biol Chem 53: 1537–1541
Taylor, JL, Jones, JDG, Sandler, S, Mueller, GM, Bedbrook, J & Dunsmuir, P (1987) Optimizing the expression of chimeric genes in plant cells. Mol Gen Genet 210: 572–577
Tracey, MV (1955) Chitinase in some Basidiomycetes. Biochem J 61: 579–589
Usai, T, Hayashi, Y, Nanjo, F, Sakai, K & Ishido, Y (1987) Transglycosylation reaction of a chitinase purified from Nocardia orientalis. Biochem Biophys Acta 923: 302–309
Usai, T, Matsui, M & Isobe, K (1990) Enzymic synthesis of useful chito-oligosaccharides utilizing transglycosylation by chitinolytic enzymes in buffer containing ammonium sulfate. Carb Res 202: 65–78
Walker, AN, Garner, RE & Horst, MN (1990) Immunocytochemical detection of chitin in Pneumocystis carinii. Infect Immun 58: 412–415
Wessels, JGH (1988) A steady state model for apical wall growth. Acta Bot Neerl 37: 3–16
Wolkin, RH & Pate, JKL (1985) Selection for nonadherent or nonhydrophobic mutants co-selects for non-spreading mutants of Cytophage johnsonae and other gliding bacteria. J Gen Microbiol 131: 7737–750
Wortman, AT, Somerville, CC & Colwell, RR (1986) Chitinase determinants of Vibrio vulnificus: Gene cloning and applications of a chitinase probe. Appl Environ Microbiol 52: 142–145
Yabuki, M, Kasai, Y, Ando, A & Fujii, T (1984) Rapid method for converting fungal cells into protoplasts with a high regeneration frequency. Exp Mycol 8: 386–390
Zarain-Herzberg, A & Arroya-Begovich, A (1983) Chitinolytic activity from Neurospora crassa. J Gen Microbiol 129: 3319–3326
Zhloba, NM, Tiunova, NA & Sidorova II (1980) extracellular hydrolytic enzymes of mycophilic fungi. Mikol Fitopatol 14: 496–499
Zikakis, J (1984) Chitin, chitosan and Related Enzymes. Academic Press, Orlando
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gooday, G.W. Physiology of microbial degradation of chitin and chitosan. Biodegradation 1, 177–190 (1990). https://doi.org/10.1007/BF00058835
Issue Date:
DOI: https://doi.org/10.1007/BF00058835