Abstract
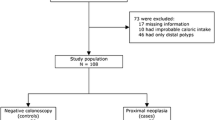
Hyperplastic polyps of the colon reveal a geographic distribution similar to that of colorectal cancer and adenomatous polyps. However, unlike adenomas—known precursors of colorectal cancer—little is known about the etiology or clinical significance of the hyperplastic polyp. In this prospective study, we set out to determine the main dietary and other lifestyle factors in the United States that might be associated with this lesion. Hyperplastic polyps of the distal colon and rectum were diagnosed in 219 of 12,922 men of the Health Professionals Follow-up Study having had an endoscopic procedure between 1986 and 1992, and 175 of 15,339 women of the Nurses' Health Study who had undergone an endoscopy for a variety of reasons between 1980 and 1990. After adjusting for age, family history of colon cancer, history of previous endoscopy, and total energy intake using multiple logistic regression, those consuming 30 g or more of alcohol per day were at increased risk relative to nondrinkers among men (relative risk [RR]=1.69; 95 percent confidence interval [CI]=1.01–2.80) and women (RR=1.79, CI=1.02–3.15). Current smoking also was found to be associated strongly positively with hyperplastic polyps in men (RR=2.45, CI=1.59–3.75) and women (RR=1.96, CI=1.16–2.86). High intake of folate was associated inversely with risk in both men (RR=0.74, CI=0.49–1.11, between high and low intakes of folate) and women (RR=0.45, CI=0.28–0.74, between high and low intakes of folate). Among macronutrients, a suggestive increase in risk existed with intake of animal fat, although this was attenuated in the full multivariate model (RR[men]=1.48, CI=0.94–2.41, and RR [women]=1.22, CI=0.77–1.94) between high and low quantities of animal fat intake. These prospective data provide evidence of associations between low folate intake, alcohol consumption, and current cigarette smoking, and risk of hyperplastic polyps of the distal colon and rectum. These same factors also have been found to be related to adenoma and cancer of the colon. The hyperplastic polyp is an indicator of populations at high risk for colorectal carcinoma, and it also may serve as a marker for factors that influence neoplastic evolution.
Similar content being viewed by others
References
Fenoglio-Preiser CM. Polyps and the subsequent development of carcinoma of the colon ad rectum: definitions and hints on tissue handling. In: Feneglio-Preiser CM, Rossini FD, eds. Adenomas and Adenomas Containing Carcinoma of The Large Bowel: Advances in Diagnosis and Therapy. New York, NY (USA): Raven Press, 1985: 15–29.
Fenoglio CM, Pascal RR. Colorectal adenomas and cancer. Pathologic relationships. Cancer 1982; 50: 2600–8.
Gillbertsen VA. Proctosigmoidoscopy and polypectomy in reducing the incidence of rectal cancer. Cancer 1974; 34: 936–43.
Arthur JF. Structure and significance of metaplastic nodules in the rectal mucosa. J Clin Pathol 1968; 21: 735–43.
Fenoglio CM, Lane N. The anatomical precursor of colorectal carcinoma. Cancer 1974; 34: 819–23.
Provenzale D, Martin ZZ, Holland KL, et al. Colon adenomas in patients with hyperplastic polyps. J Clin Gastroenterol 1988; 10: 46–9.
Ansher AF, Lewis JH, Fleischer DE, et al. Hyperplastic colonic polyps as a marker for adenomatous colonic polyps. Am J Gastroenterol 1989; 84: 113–7.
Jass JR. Nature and clinical significance of colorectal hyperplastic polyp. Semin Colon Rectal Surg 1991; 2: 246–52.
Jass JR. Relation between metaplastic polyp and carcinoma of the colorectum. Lancet 1983; i: 28–30.
Stemmermann GN, Yatani R. Diverticulosis and polyps of the large intestine. A necropsy study of Hawaii Japanese. Cancer 1973; 31: 1260–70.
Correa P. Epidemiology of polyps and cancer. In: Morson BC, ed. The Pathogenesis of Colorecial Cancer, Major Problems in Pathology, Vol. 10 Philadelphia, London, Toronto: WB Saunders, 1978: 126–52.
Haenszel W, Kurihara M. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. JNCI 1968; 40: 43–68.
Jass JR, Filipe MI, Abbas S, et al. A morphologic and histochemical study of metaplastic polyps of the colorectum. Cancer 1984; 53: 510–5.
Jass JR, Faludy KA. Immunohistoehemical demonstration of IgA and secretory component in relation to epithelial cell differentiation in normal colorearal mucosa and metaplastic polyp: A semiquantitative study. Histochem J 1985; 17: 373–80.
Hayashi T, Yatani R, Apostol J, Stemmermann GN. Pathogenesis of hyperplastic polyps of the colon: a hypothesis based on ultrastructure and in vitro cell kinetics. Gastroenterology 1974; 66: 347–56.
Czernobilsky B, Tsou KC. Adenocarcinoma, adenomas and polyps of the colon. Cancer 1968; 21: 165–77.
Stampfer MJ, Willett WC, Colditz GA, et al. A prospective study of postmenopausal estrogen therapy and coronary heart disease. N Engl J Med 1985; 313: 1044–9.
Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985; 122: 51–65.
Rimm EB, Stampfer MJ, Colditz GA, et al. Effectiveness of various mailing strategies among nonrespondents in a prospective cohort study. Am J Epidemiol 1990; 131: 1068–71.
Giovannucci E, Stampfer MJ, Colditz GA, et al. Relation of diet to the risk of colorectal adenoma in men. JNCI 1992; 84: 91–8.
Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol 1988; 127: 188–99.
Rimm EB, Giovannucci E, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992; 135: 1114–26.
United States Department of Agriculture. Agriculture Handbook No. 8 Series. Composition of Food: Raw, Processed and Prepared. Washington, DC: US Government Printing Office, 1963–88.
Willett WC. The search for the causes of breast and colon cancer. Nature 1989; 339: 389–94.
Giovannucci E, Colditz GA, Stampfer MJ, et al. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol 1991; 133: 810–7.
Clark JC, Collan Y, Eide TJ, et al. Prevalance of polyps in an autopsy series from areas with varying incidence of large bowel cancer. Int J Cancer 1985; 36: 179–86.
Jass JR, Young PJ, Robinson EM. Predictors of presence, multiplicity, size and dysplasia of colorectal adenomas. A necropsy study in New Zealand. Gut 1992; 33: 1508–13.
Hoff G, Vatn M. Epidemiology of polyps in the rectum and sigmoid colon: endoscopic evaluation of size and location of polyps. Scand J Gastroenterol 1985; 20: 356–60.
Freudenheim JL, Graham S, Marshall JR, et al. Folate intake and carcinogenesis of the colon and rectum. Int J Epidemiol 1991; 20: 368–74.
Giovannucci E, Stampfer MJ, Colditz GA, et al. Folats, methionine, and alcohol intake and risk of colorectal adenoma. JNCI 1993; 85: 875–84.
National Academy Press. Recommended Dietary Allowances, 10th Edition, Washington DC: NAP, 1989.
Colman N. Addition of folic acid to staple foods as a selective nutrition intervention strategy. Nutr Rev 1982; 40: 225–33.
Diamond M. Adenomas of the rectum and sigmoid in alcoholics: a sigmoidoscopic study. Am J Dig Dis 1972; 19: 47–50.
Stemmermann GN, Heilbrun LK, Nomura AMY, Association of diet and other factors with adenomatous polyps of the large bowel: a prospective autopsy study. Am J Clin Nutr 1988; 47: 312–7.
Kikendall JW, Bowen PE, Burgess MB, et al. Cigarettes and alcohol as independent risk factors for colonic adenomas. Gastroenterol 1989; 97: 660–4.
Kono S, Ikedu N, Yanai F, et al. Alcoholic beverages and adenomatous polyps of the sigmoid colon: a study of male self-defence officials in Japan. Int J Epidemiol 1990; 19: 848–52.
Honjo S, Kono S, Shinchi K, et al. Cigarette smoking, alcohol use and adenomatous polyps of the sigmoid colon. Jpn J Cancer Res 1992; 83: 806–11.
Tuyns AJ, Pequinot G, Gignoux M et al. Cancers of the digestive tract, alcohol and tobacco. Int J Cancer 1982; 30: 9–11.
Miller AB, Howe GR, Jain M, et al. Food items and food groups as risk factors in a case-control study of diet and colorectal cancer. Int J Cancer 1983; 32: 155–61.
Martinez I, Torres R, Frias Z, Colon JR, Fernandez N. Factors associated with adenocarcinoma of the large bowel in Puerto Rico. In: Birch JM, ed. Advances in Medical Oncology, Research and Education. Vol. 3. New York, NY (USA): Pergamon Press, 1979: 45–52.
Manousos O, Day NE, Trichopoulos D, et al. Diet and colorectal cancer: a case-control study in Greece. Int J Cancer 1983; 32: 1–5.
Klatsky AL, Armstrong MA, Friedman GD, et al. The relations of alcoholic beverage use to colon and rectal cancer. Am J Epidemiol 1988; 128: 1007–15.
Wu AH, Paganini-Hill A, Ross PK, et al. Alcohol, physical activity and other risk factors for colorectal cancer: A prospective study. Br J Cancer 1987; 55: 687–94.
Hirayama T. Association between alcohol consumption and cancer of the sigmoid colon: observations from a Japanese cohort study. Lancet 1989; ii: 725–7.
Stemmermann GN, Nomura AMY, Chyou PH, et al. Prospective study of alcohol intake and large bowel cancer. Dig Dis Sci 1990; 35: 1414–20.
Garland C, Shekelle RB, Barrett-Connor E, et al. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet 1985; i: 307.
Williams RR, Horm JW. Association of cancer sites with tobacco and alcohol consumption and socioeconomic status of patients: interview study from the third National Cancer Survey. JNCI 1977; 58: 525–47.
Bingham S. Diet and large bowel cancer. J Royal Soc Med 1990; 83: 420–2.
International Agency for Research on Cancer. Allyl and Allylic Compounds, Aldehydes, Epoxides and Peroxides. Lyon, France: IARC, 1985; IARC Monogr Eval Carcinog Risk Humans, Vol. 36.
Seitz HK, Simanowski UA, Garzon FT, et al. Possible role of acetaldehyde in ethanol-related rectal carcinogenesis in the rat. Gastroenterol 1990; 98: 406–13.
Simanowski UA, Seitz HK, Baier B, et al. Chronic alcohol consumption selectively stimulates rectal cell proliferation in the rat. Gut 1986; 27: 278–82.
Herbert VD, Colman N. Folic acid and vitamin B12. In: Shils ME, Young VR, Modern Nutrition in Health and Disease. 7th Ed. Philiadelphia, PA (USA): Lea and Febiger, 1988: 388–416.
Shaw S, Jayatillek E, Herbert V, Colman N. Cleavage of folates during ethanol metabolism. Biochem J 1989; 257: 277–80.
Barak AJ, Beckenhaur HC, Tuma DJ, Halsted CH. Effects of prolonged ethanol feeding on methionine metabolism in rat liver. Biochem Cell Biol 1987; 65: 230.
Hoff G, Vatn MH, Larsen S. Relationship between tobacco smoking and colorectal polyps. Scand J Gastroenterol 1987; 22: 13–6.
Giovannucci E, Rimm EB, Stampfer MJ, et al. A prospective study of cigarette smoking and risk of colorectal adenoma and cancer in men. JNCI 1994; 86: 183–91.
Vatn MH, Stalsberg H. The prevalence of polyps of the large intestine in Oslo: An autopsy study. Cancer 1982; 49: 819–25.
Eide TJ, Stalsberg H. Polyps of the large intestine in Northern Norway. Cancer 1978; 42: 2839–48.
Additional information
Drs Giovannucci, Stampfer, Colditz, and Willett are with the Channing Laboratory, Department of Medicine, Harvard Medical School and Brigham and Women's Hospital, Boston, MA, USA. Authors also are affiliated with: the Department of Nutrition, Harvard School of Public Health, Boston, MA (Drs Kearney, Rimm, Stampfer, Ascherio, and Willett); the Department of Epidemiology, Harvard School of Public Health (Drs Rimm, Stampfer, Colditz, Ascherio, and Willett); and the Department of Surgery, New England Deaconess Hospital, Boston, MA (Dr Bleday). Address correspondence to Dr Giovannucci, Channing Laboratory, 180 Longwood Avenue, Boston, MA 02115, USA. This project was supported by research grants number CA 55075 and HL 35464 from the National Institutes of Health and Special Institution Grant No. 18 from the American Cancer Society. Dr Colditz. was supported by a Faculty Research Award (FRA-398) from the American Cancer Society.
Rights and permissions
About this article
Cite this article
Kearney, J., Giovannucci, E., Rimm, E.B. et al. Diet, alcohol, and smoking and the occurrence of hyperplastic polyps of the colon and rectum (United States). Cancer Causes Control 6, 45–56 (1995). https://doi.org/10.1007/BF00051680
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00051680