Abstract
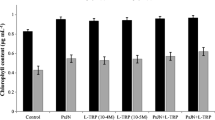
Soil microorganisms are capable of producing auxins in the presence of the physiological precursor, L-tryptophan (L-TRP). This study was designed to assess the influence of L-TRP on radish (Raphanus sativus) yield when applied to soil. The amount of L-TRP added to soil to give optimum radish growth in glasshouse studies was 3.0 mg kg-1 soil which enhanced the root yield by 1.31-fold over the control. The root/shoot ratio was increased by 1.10-fold upon this amendment. One L-TRP application was sufficient to promote growth. The best time to apply L-TRP was at the onset of seedling emergence. The application of L-TRP promoted radish yield comparable to those plants treated with indole-3-acetic acid, indole-3-acetamide and indole-3-lactic acid. Foliar application of L-TRP had no effect on the root and shoot dry weight. A field study was conducted in which L-TRP applications at a rate of 20.4 and 204 mg m-2 significantly enhanced the radish yield in fertilized plots receiving fertilization. The shoot dry weight was increased by 1.29-fold and the root dry weight by 1.15-fold over the control in response to 20.4 mg L-TRP m-2. These findings indicate that L-TRP, applied at the appropriate times and concentrations, can increase radish yield. The effect of L-TRP on radish growth could be attributed to i) substrate-dependent auxin production in soil by the indigenous microflora, ii) uptake directly by plant roots followed by metabolism within their tissues, and/or iii) a change in the balance of rhizosphere microflora affecting plant growth.
Similar content being viewed by others
References
Arshad M and Frankenberger W TJr 1990a Response of Zea mays and Lycopersicon esculentum to the ethylene precursors, L-methionine and L-ethionine applied to soil. Plant and Soil 122, 219–227.
Arshad M and Frankenberger W TJr 1990b Microbial production of plant growth regulators. In Soil Microbial Technologies. Ed. B Metting. Marcel Dekker, Inc., New York, NY. (In press).
Arshad M and Frankenberger W T Jr 1990c Microbial production of plant hormones. Plant and Soil vol., pages.
Azcon R, Aguilar C A-G D and Barea J M 1978 Effects of plant hormones present in bacterial cultures on the formation and responses to VA endomychorrhiza. New Phytol. 80, 359–364.
Brenner M L 1987 The role of hormones in photosynthate partitioning and seed filling. In Plant Hormones and Their Role in Plant Growth and Development. Ed. P J Davies. pp 474–493. Martinus Nijhoff Publishers, Dordrecht, The Netherlands.
Brown M E, Jackson R M and Burlingham S R 1968 Effects produced on tomato plants, Lycopersicum esculentum, by seed or root treatment with gibberellic acid and indole-3yl-acetic acid. J. Expt. Bot. 19, 544–552.
Chandramohan D and Mahadevan A 1968 Indole acetic acid metabolism in soils. Curr. Sci. 37 112–113.
Davies P J 1987 The plant hormones: Their nature, occurrence, and functions. In Plant Hormones and Their Role in Plant Growth and Development. Ed. P J Davies. pp 1–11. Martinus Nijhoff Publishers, Dordrecht, The Netherlands.
Frankenberger W TJr and Brunner W 1983 Method of detection of auxin-indole-3-acetic acid in soils by high performance liquid chromatography. Soil Sci. Soc. Am. J. 47, 237–241.
Frankenberger W T Jr and Fitzpatrick K L 1984 Exogenous hormone production in soil-root systems. In Improving Efficiencies in Crop Production Systems. pp 58–61. Proc. Calif. Plant Soil Conf., Am. Soc. Agron.
Frankenberger W TJr and Poth M 1987a Biosynthesis of indole-3-acetic acid by the pine ectomycorrhizal fungus, Pisolithus tinctorius. App. Environ. Microbiol. 53, 2908–2913.
Frankenberger W TJr and Poth M 1987b Determination of substituted indole derivatives by ion suppression-reversephase high-performance liquid chromatography. Anal. Biochem. 165, 300–308.
Frankenberger W TJr and Poth M 1988 L-Tryptophan transaminase of a bacterium isolated from the rhizosphere of Festuca octoflora (Graminae). Soil Biol. Biochem. 20, 299–304.
Hamence J H 1946 The determination of auxins in soils including a note on synthetic substances. Analyst 71, 111–116.
Hubbell D H, Tien T M, Gaskins M H and Lee J 1979 Physiological interaction in the Azospirillum-grass root association. In Associative N2-Fixation, Vol. I, pp 1–6. Eds. P B Vose and A P Ruschel. CRC Press, Inc., FL.
Lynch J M 1976 Products of soil microorganisms in relation to plant growth. CRC Critical Rev. Microbiol. 5, 67–107.
Lynch J M 1985 Origin, nature and biological activity of aliphatic substances and growth hormones found in soil. In Soil Organic Matter and Biological Activity. Eds. D Vaughan and R E Malcolm. pp 151–174. Martinus Nijhoff/Dr. W. Junk Publishers, Dordrecht, The Netherlands.
Narayanaswami R and Veerraju V 1969 IAA synthesis in paddy soils as influenced by ammonium sulfate fertilization. Curr. Sci. 38, 517–518.
Purushothaman D, Balaraman K and Oblisami G 1973 Indole acetic acid metabolism in soil as influenced by pesticide application. Curr. Sci. 42, 365–366.
Purushothaman D, Marimuthu T, Venkataramanan C V and Kesavan R 1974 Role of actinomycetes in the biosynthesis of indole acetic acid in soil. Curr. Sci. 43, 413–414.
Rovira A D and McDougall B M 1967 Microbiological and biochemical aspects of the rhizosphere. In Soil Biochemistry. Eds. A D McLaren and G H Peterson. pp 417–463. Marcel Dekker, Inc., New York.
Sheldrake A R 1971 The occurrence and significance of auxin in the substrate of bryophytes. New Phytol. 70, 519–526.
Stevenson F J 1982 Organic forms of organic nitrogen. In Nitrogen in Agricultural Soils. Ed. F J Stevenson. pp 67–122. Agronomy No. 22, Am. Soc. Agron., Madison, WI.
Stewart W S and Anderson M S 1942 Auxins in some American soils. Bot. Gaz. 103, 570–575.
Tien T M, Gaskins M H and Hubbell D H 1979 Plant growth substances prdouced by Azospirillum brasilense and their effect on the growth of pearl millet (Pennisetum americanum L.). Appl. Environ. Microbiol. 37, 1016–1024.
Whitehead D C 1963 Some aspects of the influence of organic matter on soil fertility. Soil Fertil. 26, 217–223.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Frankenberger, W.T., Chang, A.C. & Arshad, M. Response of Raphanus sativus to the auxin precursor, L-tryptophan applied to soil. Plant Soil 129, 235–241 (1990). https://doi.org/10.1007/BF00032418
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00032418