Abstract
Effect of single and simultaneous addition of Ni and Co to Ti45Zr5Pdx on the phase transformation and strain recovery of the alloys was investigated to understand multi-component effect on high-temperature shape memory alloys. The martensitic transformation from B2 to B19 structures occurred in Ti45Zr5Pd40Ni10, Ti45Zr5Pd40Co10, Ti45Zr5Pd40Ni8Co2, and Ti45Zr5Pd40Ni2Co8. In contrast, at room temperature, martensitic transformation did not occur and the B2 structure was stable in Ti45Zr5Pd30Ni20 and Ti45Zr5Pd40Ni5Co5. This is due to the effect of Ni for Ti45Zr5Pd30Ni20 and the effect of multi-component for Ti45Zr5Pd40Ni5Co5. The shape memory effect was investigated using a thermal cyclic test. The perfect shape recovery was obtained after the repeated thermal cyclic test, indicating the potential of TiZrPd (Ni, Co) alloys as shape memory alloys.
Similar content being viewed by others
Introduction
The shape memory effect (SME) occurs by reverse martensitic transformation (MT). TiNi is the most commonly used shape memory alloy (SMA), but the martensitic transformation temperature (MTT) of TiNi from the B2 to B19 structures is lower than 100 °C. To expand the applications of SMAs, the MTTs should be increased to raise the operation temperatures of SMAs [1]. Thus, various techniques have been employed to raise the MTT of TiNi, such as the addition of alloying elements such as Pt, Pd, Hf, and Zr into TiNi.
Recently, Hf-added TiNi alloys have attracted attention due to their high strength by nanoprecipitates and low cost; however, their MTTs are between 100 and 200 °C [2,3,4,5,6,7,8,9,10]. The most attractive high-temperature shape memory alloy composition is Ni50.3Ti29.7Hf20, and the perfect shape recovery was obtained even under 500–700 MPa with a large work output, ~ 20 J/cm3, equivalent to that of TiNi. In addition, Hf raised the MTTs of TiNi alloys, and the highest martensite start temperature (Ms) was found as ~ 500 °C for Ni49.8Ti20.2Hf30 [10], indicating the potential of TiNi-Hf alloys with nanoprecipitates as high-temperature shape memory alloys (HT-SMAs).
High-entropy alloys (HEAs) have attracted attention for HT-SMA applications [11,12,13,14]. HEAs are multi-component equiatomic or near-equiatomic alloys and are expected to function as the next-generation structural materials. HEAs are also expected as high-temperature materials since they have severe lattice distortion and sluggish diffusion. For example, Ti16.667Zr16.667Hf16.667Ni25Cu25 [11] was the first attempt of HEAs as SMAs. It was found that Ti16.667Zr16.667Hf16.667Ni25Cu25, as well as TiNi, had the MT from B2 to B19, and the austenite finish temperature (Af) was 337 °C, and the martensite finish temperature (Mf) was 127 °C. The complete recovery was found between 127 and 377 °C, and the large strain recovery, approximately 4.9%, was obtained under 650 MPs due to the solid-solution hardening effect [12, 13]. The most impacted investigation was found in Ni25Pd25Ti25Hf25 and Ni25Pd25Ti16.6Hf16.6Zr16.6 [14]. Their MTs from the B2 to B19 structure as well as TiPd occurred, and their Af and As were approximately 700 and 780 °C, respectively, indicating HT-SMA characteristics [14].
We have focused on TiPd [15,16,17,18], TiAu [19, 20], and TiPt [21,22,23,24,25,26,27,28,29] as possible new HT-SMAs because MT occurs from B2 to B19 structure [30]. The MTTs of TiPd, TiAu, and TiPt are approximately 570, 600, and 1000 °C [31]. In addition to the understanding of the third element addition on the MT and the SME, we have investigated the multi-component alloys. Although their alloy compositions were not equiatomic, we considered that multi-component alloys have a similar HEA effect due to the increase in entropy compared with conventional alloys. Then, the MT and SME of the Ti–Pd–Pt–Zr quaternary alloys were investigated [32, 33]. The MTTs increased with the increase in Pt concentration, and the highest Af was obtained at 935 °C in Ti45Pt45Pd5Zr5 [32]. However, the perfect strain recovery was not obtained in all the tested alloys [32, 33]. Then, multi-component alloys, TiZrPdPtNi, were investigated to understand the multi-component effect on MT and SME [34]. The two HEAs (∆Smix = 1.5R), Ti35Zr15Pd20Ni15Pt15 and Ti40Zr10Pd20Ni15Pt15, and two medium entropy alloys (MEAs, ∆Smix = 1.3R), Ti45Zr5Pd20Ni5Pt25 and Ti45Zr5Pd20Ni10Pt20, were investigated. Here ∆Smix is the mixing entropy defined by Eq. (1).
Here, \({x}_{i}\) is the mole fraction of component I and n is the number of constituent elements. The MT was obtained only in the MEAs and not in the HEAs. The B2 structure was stable at room temperature in the HEAs. The perfect recovery was obtained in the MEAs under larger applied stress compared with the ternary TiPdZr alloys [18]. This is due to the large lattice distortion in the MEAs than the ternary alloys, also known as the multi-component effect.
A previous study showed that although Pt addition effectively increased MTTs because TiPt has MT, extremely high MTT easily introduced plastic deformation in the austenite phase [32, 33]. Then, Co was selected instead of Pt in this study because the B2 structure is stable in TiCo, and Co addition leads to no segregation or precipitation in TiPd. However, it is also expected that Co addition drastically decreases MTTs. Then, understanding of the effect of Co addition on MTT is important to design new HT-SMAs. In this study, through the investigation of TiZrPd (Ni, Co), the effect of single Ni or Co addition and simultaneous addition of Ni and Co to TiZrPd and the multi-component effect on MT and SME was investigated.
Experimental Procedure
18 g ingots with nominal compositions shown in Table 1 were prepared by arc melting in an Ar atmosphere. In this study, Ni or Co was added to replace Pd, while the concentration of Ti and Zr were held at 45 and 5 at.%. In addition to single Ni or Co addition, the simultaneous addition of Ni and Co was performed. For the simultaneous addition, the total concentration of Ni and Co were held at 10 at.% and the ratio of Ni to Co was changed.
The alloy ingots were compressed to approximately 60% of their original height at 1000 °C in the B2 region. The compressed ingots were cut into samples of 5 × 15 × 6 mm. The samples were sealed in silica tubes with argon gas and solution-treated at 1000 °C for 3 h, followed by quenching in iced water.
Samples with 4 × 4 × 1 mm dimensions were cut from the solution-treated samples, embedded in resins, and polished mechanically until the surfaces became mirror planes. Then, the microstructures were observed in backscattered mode by a scanning electron microscope (JEOL 7001F, JEOL) at an accelerating voltage of 20 kV.
Samples for differential scanning calorimetry (DSC, DSC3200s, Material Analysis and Characterization) were prepared by cutting disk-shaped specimens from the solution-treated samples. These specimens had a diameter of 4 mm, a thickness of 1 mm, and weighed approximately 100 mg. MTTs were measured in DSC with heating and cooling rates of 10 °C/min.
Plate-like samples with 10 × 15 × 1 mm dimensions were cut from the solution-treated samples, and the constituent phase was identified by X-ray diffraction (XRD) analysis (RINT-TTR III, Rigaku) using Cu-Kα radiation at 50 kV and 300 mA at room temperature.
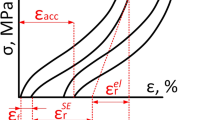
Thermal cyclic tests were performed using specimens with 3 × 3 × 5 mm dimensions in the temperature range Mf − 30 to Af + 30 °C under compressive stresses (Shimadzu AG–X, Shimadzu) to investigate the shape memory effects of the alloys. In the thermal cyclic test, the sample was first heated to Af + 30 °C, then cooled to Mf—30 °C, and again heated to Af + 30 °C. The sample length was directly measured by a CCD camera. The strain recovery and the work output were estimated using the following equations from the strain-temperature curves:
Here, εr, εt, and σa represent the recovery strain, the transformation strain, and the applied stress, respectively.
Training, i.e., repeated thermal cyclic testing under constant stress, was also applied to improve the shape recovery and investigate the stability of SME.
Results and Discussion
Microstructure and Constituent Phase
The backscattered electron images of the solution-treated alloys are shown in Fig. 1. A typical multivariant martensite twin structure was observed in Ti45Zr5Pd40Ni10, Ti45Zr5Pd40Co10, Ti45Zr5Pd40Ni8Co2, and Ti45Zr5Pd40Ni2Co8. On the other hand, the martensite twin structure was not observed in Ti45Zr5Pd30Ni20 and Ti45Zr5Pd40Ni5Co5. The microstructural observations indicate that the MT occurs in Ti45Zr5Pd40Ni10, Ti45Zr5Pd40Co10, Ti45Zr5Pd40Ni8Co2, and Ti45Zr5Pd40Ni2Co8, but it does not occur in Ti45Zr5Pd30Ni20 and Ti45Zr5Pd40Ni5Co5. Ti2Pd precipitates (a few μm in size) were formed mainly on the grain boundaries and occasionally inside the grains.
The XRD patterns obtained at room temperature are shown in Fig. 2. The B19 structure was clearly identified in Ti45Zr5Pd40Ni10, Ti45Zr5Pd40Co10, Ti45Zr5Pd40Ni8Co2, and Ti45Zr5Pd40Ni2Co8, but the B2 structure was found in Ti45Zr5Pd30Ni20 and Ti45Zr5Pd40Ni5Co5. The results of XRD agrees well with the microstructural observation that the MT occurred in Ti45Zr5Pd40Ni10, Ti45Zr5Pd40Co10, Ti45Zr5Pd40Ni8Co2, and Ti45Zr5Pd40Ni2Co8, but not in Ti45Zr5Pd30Ni20 and Ti45Zr5Pd40Ni5Co5.
MTT
The DSC curves of the alloys are shown in Fig. 3. As expected from the microstructural observation and the XRD analysis, the peaks indicating the MT was observed in Ti45Zr5Pd40Ni10, Ti45Zr5Pd40Co10, Ti45Zr5Pd40Ni8Co2, and Ti45Zr5Pd40Ni2Co8 but not in Ti45Zr5Pd30Ni20 and Ti45Zr5Pd40Ni5Co5. The As, Af, Ms, and martensite finish (Mf) temperatures obtained by DSC are summarized in Table 1 together with the temperature hysteresis between Af and Ms. For reference, the MTTs of Ti50Pd50 and Ti45Pd50Zr5 [17] are also shown in Table 1. The MTTs are plotted as the functions of Ni and Co concentration in Fig. 4. Compared with Ti45Pd50Zr5, the MTT decreased with the single addition of Ni or Co or the simultaneous addition of Ni and Co. 1 at.% Ni addition decreased As by 27 °C, Af by 25 °C, Ms by 26 °C, and Mf by 27 °C. MTTs by the addition of 20 at.% Ni decreased to lower than 0 °C as the MTTs decreased with the same ratio up to 20 at.%. Then, it is reasonable that no MT was observed in Ti45Zr5Pd30Ni20. On the other hand, 1 at.% Co addition decreased As by 19 °C, Af by 15 °C, Ms by 13 °C, and Mf by 15 °C. The decreases in MTTs by Co addition were smaller than those by the addition of Ni. This is not expected since no MT takes place in TiCo, and the B2 structure is much more stable in TiCo than in TiNi. It is expected that Co addition decreases MTTs more than Ni addition, but the opposite trend occurs in the multi-component alloys. Interestingly, the MTTs of the alloy with 8 Ni and 2 Co were higher than those of the alloy with 2 Ni and 8 Co. Moreover, no MT was observed in Ti45Zr5Pd40Ni5Co5. Since the total addition of Ni and Co were 10 at.% for Ti45Zr5Pd40Ni8Co2, Ti45Zr5Pd40Ni5Co5, and Ti45Zr5Pd40Ni2Co8, it is considered that the MTTs of Ti45Zr5Pd40Ni5Co5 are between those of Ti45Zr5Pd40Ni8Co2 and Ti45Zr5Pd40Ni2Co8. However, the B2 structure was stable at room temperature, and the MT was not observed in Ti45Zr5Pd40Ni5Co5. The mixing entropy effect is similar to HEAs when the amount of the alloying element is equivalent. In the case of Ti45Zr5Pd40Ni5Co5, the amount of Zr, Ni, and Co were 5 at.%, and the mixing entropy shown in Eq. (1) was slightly higher, 1.18R than other multi-component alloys, 1.16R. In HEA, for example, the MT occurred, and the B19 structure appeared at room temperature in Ti16.667Zr16.667Hf16.667Co25Ni25 with a mixing entropy of 1.23R. On the other hand, no MT was found, and the B2 structure appeared at room temperature in Ti16.667Zr16.667Hf16.667Co16.667Ni16.667Cu16.667 with equiatomic composition and the mixing entropy of 1.47R [11]. The equivalent addition of the alloying elements causes partially large lattice distortion and restrains MT, as observed in partially equivalent TiPdZrV [18]. The MTTs of Ti45Pd50Zr5 and Ti45Pd50V5 were approximately 450 °C. The simultaneous addition of Zr and V while keeping the total composition at 5 at.%, decreased Ms and Mf. Ms and Mf were the lowest in Ti45Pd50Zr2.5V2.5 with the equivalent amount of Zr and V and the slightly higher mixing entropy of 0.89 R compared with Ti45Pd50Zr4V1 and Ti45Pd50Zr1V4 with a mixing entropy of 0.88 R. In TiZrPdNiPt, the B2 structure was stable, and no MT was found in HEAs, such as Ti35Zr15Pd20Ni15Pt15 and Ti40Zr10Pd20Ni15Pt15 with the mixing entropy of 1.5R [34]. On the other hand, in the MEA with the smaller mixing entropy of 1.3R and 1.4R than HEA, Ti45Zr5Pd20Ni5Pt25 and Ti45Zr5Pd20Ni10Pt20, the MT was observed. Although the absolute value of the mixing entropy does not indicate the trend of MTTs in general, when comparing across the same combinations of alloying elements, a higher mixing entropy indicates that MTs are less likely.
MTT and temperature hysteresis of tested alloys together with Ti45Zr5Pd50 [17] as a function of Ni and Co concentration. Martensite start (Ms), martensite finish (Mf), austenite start (As), and austenite finish (Af) temperatures are shown
The temperature hysteresis between Af and Ms of the tested alloys were all between 40 °C and 61 °C, which is acceptable for actuators. As shown in Fig. 4, the temperature hysteresis of TiPdZrNiCo was larger than those of TiZrPdNi and TiZrPdCo. Generally, high-entropy SMAs have a larger temperature hysteresis than conventional SMAs because the severe lattice distortion restrains MT and reverse MT. High-entropy SMAs have lower Ms and higher As than those of conventional SMAs [11,12,13,14]. In our case, we believe that slightly higher mixing entropy caused larger lattice distortions and the MT became more difficult, resulting in large temperature hysteresis.
SME
The strain-temperature curves based on thermal cyclic testing are shown in Fig. 5. The applied stress ranged from 15 to 200 MPa. In Ti45Zr5Pd40Ni10, the perfect strain recovery was obtained for up to 50 MPa, and a small irrecoverable strain was observed over 100 MPa, as shown in Fig. 5a. A similar behavior was observed in multi-component alloys, Ti45Zr5Pd40Ni8Co2, and Ti45Zr5Pd40Ni2Co8, shown in Fig. 5c, d. However, a significant difference compared with Ti45Zr5Pd40Ni10 is that the extra cooling was necessary to return to the original position during cooling, as shown by the arrows. It means that the actual temperature hysteresis of the multi-component alloys was over 100 °C. In Ti45Zr5Pd40Co10, the transformation strain was much smaller, < 1% compared with the other three alloys, and the perfect strain recovery was obtained for up to 200 MPa.
The transformation, the recoverable and irrecoverable strains were estimated from the strain-temperature curves in Fig. 5 and plotted, as shown in Fig. 6a, b. The transformation strains of Ti45Zr5Pd40Ni10, Ti45Zr5Pd40Ni8Co2, and Ti45Zr5Pd40Ni2Co8 at 200 MPa were between 3.5 and 6%. On the other hand, the transformation strain of Ti45Zr5Pd40Co10 was approximately 0.5% at 200 MPa in Fig. 6a. This indicates that Co reduced the unit cell volume difference between martensite and austenite. In the previous study, the transformation strain was approximately 5% for Ti45Zr5Pd50 [33], 4.5% for Ti45 Zr5Pd45Pt5 [33], 3% for Ti45 Zr5Pd25Pt5 [33], 2% for Ti45Zr4V1Pd50 [18], and 3% for Ti45Zr5Pd20Ni5Pt25 [34]. Expect for Ti45Zr5Pd40Co10, the alloys in the present study have relatively large transformation strain. The transformation strains of Ti45Zr5Pd40Ni8Co2, and Ti45Zr5Pd40Ni2Co8 were larger than that of Ti45Zr5Pd40Ni10, indicating the effect of multi-component alloying. However, the highest three transformation strains were obtained in the alloy with 8 at.% Co, 2 at.% Co, and 0 at.% Co, respectively, even though the smallest transformation strain was obtained in Ti45Zr5Pd40Co10 with 10 at.% Co. The reason is not clear. The respective order of the recoverable strain was the same as the transformation strain, as shown in Fig. 6b. The irrecoverable strains of all the tested alloys were smaller than 0.5% for up to 200 MPa, and the irrecoverable strain was 0 in Ti45Zr5Pd40Co10. They were smaller than those of other TiPd alloys, which range approximately 1–2% [18, 33, 34]. Then, the strain recovery was 100% for Ti45Zr5Pd40Co10 and over 80% for the other three alloys, as shown in Fig. 6c. The work outputs of the tested alloys, expect for Ti45Zr5Pd40Co10, were between 6 and 10 J/cm3 at 200 MPa, as shown in Fig. 6d. They are comparable with the work output of Ti45Zr5Pd50 [33] and larger than those of TiZrPdPt [33], TiZrVPd [18], and TiZrPdNiPt [34].
Training Effect
Since the irrecoverable strain was obtained, training, that is, the repeated thermal cyclic tests under constant stress, was performed for Ti45Zr5Pd40Ni10 and Ti45Zr5Pd40Co10 to improve SME and investigate their stability. The strain-temperature curves of Ti45Zr5Pd40Ni10 for 100 cycles under 300 MPa are shown in Fig. 7a. The initial transformation strain was around 5.6%, and the irrecoverable strain was demonstrated for the first cycle. Figure 7b indicates the strain-temperature curves of the first nine cycles during which the irrecoverable strain decreased. The perfect recovery was achieved after 20 cycles. The strain-temperature curves of the last nine cycles and comparison of the first and the last nine cycles are shown in Fig. 7c, d, respectively. The nine strain-temperature curves, these traces align almost exactly in Fig. 7c. This indicates that the stable, perfect recovery was obtained under 300 MPa, although the transformation strain became smaller, 3.6%. The work output for the perfect recovery in the last nine cycles was evaluated as 10.8 J/cm3. The shape of the strain-temperature curves of the first cycle and the last nine cycles was drastically changed, as shown in Fig. 7d, that is, As and Af did not change during the thermal cyclic test, but Ms and Mf increased during the thermal cyclic test. Temperature hysteresis became smaller in the last nine cycles compared with the first cycle, as shown in Fig. 7d. The obtained MTTs are summarized in Table 2.
The training was performed under 700 MPa for Ti45Zr5Pd40Co10, and the strain-temperature curves for nine cycles are shown in Fig. 8a. The irrecoverable strain was observed for the first curve, as shown in Fig. 8b, but it disappeared after five cycles. As shown in Fig. 8c, the work output was approximately 7 J/cm3 for the 9th cycle. In the case of Ti45Zr5Pd40Co10, MTT was approximately constant during the thermal cyclic test, as shown in Fig. 8a and Table 2. However, the decrease in the transformation strain was observed in Fig. 8 that is; 1.3% for the first cycle and 1% for the 9th cycle.
The irrecoverable strain is introduced in two ways during the thermal cyclic test. The first is via the plastic deformation of the austenite phase when the temperature reaches above Af. The second is via the dislocations formed on the habit plane between the austenite and martensite phases due to the volume difference between the austenite and martensite phases during the thermal cyclic test [35]. In this case, the irrecoverable strain decreases and becomes finally negligible during the repeated thermal cyclic tests because the accumulation of dislocations introduced during phase transformation reduces the number of newly introduced dislocations. For example, the irrecoverable strain disappeared, and the perfect recovery was obtained after 100 cycles in NiTi alloys [35, 36]. The perfect recovery was finally obtained for both Ti45Zr5Pd40Ni10 and Ti45Zr5Pd40Co10, indicating that the irrecoverable strain was introduced by the phase transformation, not the plastic deformation of the austenite phase.
In both alloys, the transformation strain decreased during the thermal cyclic tests, as shown in Figs. 7 and 8. Dislocations were introduced by phase transformation and accumulated on the habit plane. The accumulated dislocations decreased the area of the martensite phase during the repeated thermal cyclic test, and as a result, the total transformation strain decreased. It is likely that the number of accumulated dislocations is less in the alloy with the small transformation strain, that is, the volume difference between the austenite and martensite phases. It is also known that MTTs are affected by applied stress during thermal cyclic testing and increase under large applied stress. It is considered that the alloy with the large transformation strain needs thermal energy more than the alloy with the small transformation strain for reverse transformation. Then, MTTs will be increased for the alloy with the large transformation strain, but MTTs are not drastically changed for the alloy with the small transformation strain. This is the reason why MTTs became higher in Ti45Zr5Pd40Ni10, and MTTs were insensitive to doping in Ti45Zr5Pd40Co10 under the applied stress.
The work outputs of the alloys with perfect recovery are plotted in Fig. 9 as a function of As together with the previous results [5, 6, 18, 34]. It is clear that the work output drastically decreased with the increase in As. The work output of the conventionally used TiNi was between 15 and 25 J/cm3. TiNi-Hf nanoprecipitates indicate almost the same work output as TiNi [5, 6]. In our study, although As of Ti45Zr5Pd40Co10 was 303 °C and the perfect recovery was obtained, the work output was minimal due to the small recovery of strain. Ti45Zr5Pd40Co10 indicated stability under 700 MPa, but As were lower to 260 °C. As a result, the advantage as HT-SMA was not obtained. MTTs of the other alloys were ~ 200 °C. Therefore, Ni and/or Co addition to TiPd is not suitable to obtain HT-SMAs.
Conclusions
The effects of the addition of Ni and/or Co to Ti45Zr5Pdx on phase transformation and strain recovery were investigated. The MT occurred in Ti45Zr5Pd40Ni10, Ti45Zr5Pd40Co10, Ti45Zr5Pd40Ni8Co2, and Ti45Zr5Pd40Ni2Co8. However, in Ti45Zr5Pd30Ni20 and Ti45Zr5Pd40Ni5Co5, the MT did not occur and B2 structure remained at room temperature. The MTT decreased by the addition of Ni and/or Co. Ti45Zr5Pd40Co10 had the highest MTTs among the tested alloys: As, Af, Ms, and Mf were 303, 361, 339, and 298 °C, respectively. Ti45Zr5Pd40Ni2Co8 had the lowest MTTs: As, Af, Ms, and Mf were 143, 189, 135, and 108 °C, respectively. In Ti45Zr5Pd30Ni20, an addition of 1 at.% Ni caused an MTT decrease of approximately 25 °C and the addition of 20 at.% Ni decreased Af to a temperature lower than 0 °C. In Ti45Zr5Pd40Ni5Co5, the relatively large mixing entropy drastically decreased Ms, and the austenite phase becomes stable at room temperature.
The strain recovery was investigated using a thermal cyclic test at 15, 50, 100, 150, and 200 MPa. The perfect recovery was obtained up to 200 MPa in Ti45Zr5Pd40Co10, although the recovery strain was only ~ 0.4% at 200 MPa. In the other three alloys, Ti45Zr5Pd40Ni10, Ti45Zr5Pd40Ni8Co2, and Ti45Zr5Pd40Ni2Co8, the small irrecoverable strain was found. However, the repeated thermal cyclic test decreased the irrecoverable strain, until the perfect strain recovery was obtained. The stabilized cycles with perfect recovery were obtained under 300 MPa in Ti45Zr5Pd40Ni10. The work output was approximately 10.8 J/cm3 at an As of 210 °C. The perfect recovery was obtained under 700 MPa in Ti45Zr5Pd40Co10. The work output of 7 J/cm3 was obtained at an As of 260 °C.
References
Ma J, Karaman I, Noebe RD (2010) High temperature shape memory alloys. Int Mater Rev 55:257–315
Bigelow GS, Garg A, Padula SA, Gaydosh DJ, Noebe RD (2011) Load-biased shape-memory and superelastic properties of precipitation strengthened high-temperature Ni50.3Ti29.7Hf20 alloy. Scr. Mater. 64:725–728
Coughlin DR, Phillips PJ, Bigelow GS, Garg A, Noebe RD, Mills MJ (2012) Characterization of the microstructure and mechanical properties of a 50.3Ni-29.7Ti-20Hf shape memory alloy. Scr. Mater. 67:112–115
Benafan O, Noebe RD, Padula SA, Vaidyanathan R (2012) Microstructural response during isothermal and isobaric loading of a precipitation-strengthened Ni-29.7Ti-20Hf high-temperature shape memory alloy. Metall Mater Trans A 43:4539–4552
Karaca HE, Saghaian SM, Ged G, Tobe H, Basaran B, Maier HJ, Noebe RD, Chumlyakov YI (2013) Effects of nanoprecipitation on the shape memory and material properties of an Ni-rich NiTiHf high temperature shape memory alloy. Acta Mater 61:7422–7431
Benafan O, Grag A, Noebe RD, Bigelow GS, Padula SA, Gaydosh DJ, Schell N, Mabe JH, Vaidyanathan R (2014) Mechanical and functional behavior of a Ni-rich Ni 50.3 Ti 29.7 Hf 20 high temperature shape memory alloy. Intermet. 50:94–107
Shuitcev A, Vasin RN, Fan XM, Balagurov AM, Bobrikov IA, Li L, Golovin IS, Tong YX (2020) Volume effect upon martensitic transformation in Ti29.7Ni50.3Hf20 high temperature shape memory alloy. Scr. Mater. 178:67–70
Karakoc O, Hayrettin C, Evirgen A, Santamarta R, Canadinc D, Wheeler RW, Wang SJ, Lagoudas DC, Karaman I (2019) Role of microstructure on the actuation fatigue performance of Ni-Rich NiTiHf high temperature shape memory alloys. Acta Mater 175:107–120
Hayrettin C, Karakoc O, Karaman I, Mabe JH, Santamarta R, Pons J (2019) Two way shape memory effect in NiTiHf hightemperature shape memory alloy tubes. Acta Mater 163:1–13
Umale T, Salas D, Tomes B, Arroyave R, Karaman I (2019) The effects of wide range of compositional changes on the martensitic transformation characteristics of NiTiHf shape memory alloys. Scr Mater 161:78–83
Fistov GS, Kosorukova TA, Koval YN, Odnosum VV (2015) High entropy shape memory alloys. Mater Today Proc 2:S499–S503
Chen C-H, Chen Y-J (2019) Shape memory characteristics of (TiZrHf)50Ni25Co10Cu15 high entropy shape memory alloy. Scr Mater 162:185–189
Lee H-C, Chen Y-J, Chen C-H (2019) Effect of solution treatment on the shape memory functions of (TiZrHf)50Ni25Co10Cu15 high entropy shape memory alloy. Entropy 21:1027–1041
Canadinc D, Trehern W, Ma J, Karaman I, Sun F, Chaudhry Z (2019) Ultra-high temperature multi-component shape memory alloys. Scr Mater 158:82–87
Yamabe-Mitarai Y, Arockiakumar R, Wadood A, Suresh KS, Kitashima T, Hara T, Shimojo M, Tasaki W, Takahashi M, Taahashi S, Hosoda H (2015) Ti(Pt, Pd, Au) based high temperature shape memory alloys. Mater Today Proc 2:S517–S522
Arockiakumar R, Takahashi M, Takahashi S, Yamabe-Mitarai Y (2013) Microstructure, mechanical, and shape memory properties of Ti-55Pd-5x (x=Zr, Hf, V, Nb) alloys. Mater Sci Eng A 585:86–93
Kawakita M, Takahashi M, Takahashi S, Yamabe-Mitarai Y (2012) Effect of Zr on phase transformation and high-temperature shape memory effect in TiPd alloys. Mater Lett 89:336–338
Sato H, Kim H-Y, Shimojo M, Yamabe-Mitarai Y (2017) Training effect on microstructure and shape recovery in Ti-Pd-Zr Alloys. Mater Trans 58(10):1479–1486
Wadood A, Hosoda H, Yamabe-Mitarai Y (2014) Phase transformation, oxidation, and shape memory properties of Ti-50Au-10Zr alloy for high temperature applications. J Alloys Compd 595:200–205
Wadood A, Yamabe-Mitarai Y (2015) Silver- and zirconium-added ternary and quaternary TiAu based high temperature shape memory alloys. J Alloy Compd 646:1172–1177
Yamabe-Mitarai Y, Hara T, Miura S, Hosoda H (2006) Mechanical properties of Ti-50(Pt, Ir) high-temperature shape memory alloys. Mat Trans 47(3):650–657
Nishida M, Matsuda M, Yasumoto Y, Yano S, Yamabe-Mitarai Y, Hara T (2008) Crystallography and morphology of twins in equiatomic TiPt martensite. Mater Sci Technol 24(8):884–889
Yamabe-Mitarai Y, Hara T, Miura S, Hosoda H (2010) Shape memory effect and pseudoelasticity of TiPt. Intermet 18:2275–2280
Yamabe-Mitarai Y, Hara T, Miura S, Hosoda H (2012) Phase transformation and shape memory effect of Ti(Pt, Ir). Metall Trans A 43A:2901–2911
Wadood A, Takahashi M, Takahashi S, Hosoda H, Yamabe-Mitarai Y (2013) High-temperature mechanical and shape memory properties of TiPt-Zr and TiPt-Ru alloys. Mater Sci Eng A 564:34–41
Yamabe-Mitarai Y, Hara T, Kitashima T, Miura S, Hosoda H (2013) Composition dependence of phase transformation behavior and shape memory effect of Ti(Pt, Ir). J Alloys Compd 577S:S399–S403
Wadood A, Yamabe-MItarai Y (2014) Recent research developments related to near-equiatomic TiPt alloys for high-temperature (above 800 °C) applications. Platin Met Rev 58(2):61–67
Wadood A, Yamabe-Mitarai Y (2014) TiPd-Co and TiPt-Ru high temperature shape memory alloys. Mater Sci Eng A 610:106–110
Yamabe-Mitarai Y, Wadood A, Arockiakumar R, Hara T, Takahashi M, Takahashi S, Hosoda H (2014) Phase transformation and shape memory effect of Ti(Pt, Ir). Mater Sci Forum 783–786:2541–2545
Donkersloot HC, Van Vucht JHN (1970) Martensitic transformations in gold-titanium, palladium-titanium and platinum-titanium alloys near the equiatomic composition. J Less Common Met 20(2):83–91
Villars P, Okamoto H, Cenzual K (eds) (2006) ASM Alloy Phase Diagrams Center https://www1.asminternational.org/AsmEnterprise/APD, ASM International, Materials Park, OH
Yamabe-Mitarai Y, Takebe W, Shimojo M (2017) Phase transformation and shape memory effect of Ti-Pd-Pt-Zr high temperature shape memory alloys. Shape Mem Superelast 3(4):381–391
Tasaki W, Shimojo M, Yamabe-Mitarai Y (2019) Thermal cyclic properties of TI-Pd-Pt-Zr high-temperature shape memory alloys. Crystals 9:595–611
Matsuda H, Sato H, Shimojo M, Yamabe-Mitarai Y (2019) Improvement of high-temperature shape-memory effect by multi-component alloying for TiPd alloys. Mater Trans 60(11):2281–2291
Atli KC, Karaman I, Noebe RD, Gaydosh D (2013) The effect of training on two-way shape memory effect of binary NiTi and NiTi based ternary high temperature shape memory alloys. Mater Sci Eng A 560:653–666
Atli KC, Franco BE, Karaman I, Gaydosh D, Noebe RD (2013) Influence of crystallographic compatibility on residual strain of TiNi based shape memory alloys during thermo-mechanical cycling. Mater Sci Eng A 574:9–16
Acknowledgements
The work was performed under NIMS project and internship program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This invited article is part of a special issue of Shape Memory and Superelasticity to honor Prof. Dr.-Ing. Gunther Eggeler. This special issue was organized by Prof. Hüseyin Sehitoglu, University of Illinois at Urbana-Champaign, and Prof. Dr.-Ing. Hans Jürgen Maier, Leibniz Universität Hannover.).
Rights and permissions
About this article
Cite this article
Yamabe-Mitarai, Y., Ohl, B., Bogdanowicz, K. et al. Effects of Ni and Co on Phase Transformation and Shape Memory Effect of Ti–Pd–Zr Alloys. Shap. Mem. Superelasticity 6, 170–180 (2020). https://doi.org/10.1007/s40830-020-00286-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40830-020-00286-y