Abstract
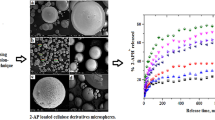
The aim of this study was to investigate the combined influence of 3 independent variables in the preparation of sustained release Nateglinide (NTG) microspheres by O/W solvent emulsification method. A 3-factor, 3-level Box–Behnken design was used to derive second order polynomial equation and construct contour plots to predict responses. The independent variables selected were polymer concentration (A), surfactant concentration (B) and speed of the stirrer (C). Percentage drug loading (Y1) and Percentage drug release (Y2) were considered dependent variables. The prepared microspheres were evaluated for percentage of yield, drug loading, drug release study in 6.8 phosphate buffer, Fourier transform infrared (FT-IR), X-ray diffraction (XRD), scanning electron microscopy analysis. Contour plots were constructed to show the effects of of A, B, C on Y1 and Y2. The yield of microspheres was found to be in the range of 42.29–97.22 %. The drug loading was found to be in the range of 12.18 % (F9) to 24.55 % (F14). FT-IR analysis revealed no drug excipient interference. The morphology of evaluated microspheres were found to be spherical and smooth in nature. In XRD analysis crystalline pattern of pure NTG was changed to amorphous pattern when converted to microspheres. Out of 17 batches, formulation batches F1, F4, F12, F14, F17 had percentage drug loading 52.75, 50.78, 43.88, 47.45, 44.78 % at 10 h respectively which indicated excellent sustained drug release pattern. From this study it was concluded that NTG loaded ethyl cellulose sustained release microspheres were developed using 3-factor, 3-level Box–Behnken design.










Similar content being viewed by others
References
Akiyoshi M, Kakei M, Nakazaki M, Tanaka H (1995) A new hypoglycemic agent, A-4166, inhibits ATP-sensitive potassium channels in rat pancreatic beta-cells. Am J Physiol 268:E185–E193
Box GEP, Behnken DW (1960) Some new three level designs for the study of quantitative variables. Technometrics 2:455–475
Davis SS, Hardy JG, Taylor MJ, Whalley DR, Willson CG (1984) A comparative study of gastrointestinal transit of a pellet and tablet formation. Int J Pharm 21:167–177
Fujitani S, Yada T (1994) A novel d-phenylalanine-derivative hypoglycemic agent A-4166 increases cytosolic free Ca2+ in rat pancreatic beta-cells by stimulating Ca2+ influx. Endocrinology 134:1395–1400
Gohel MC, Amin AF (1998) Formulation optimization of controlled release diclofenac sodium microspheres using factorial design. J Control Release 51:115–122
Guyot M, Fawaz F (1998) Nifedipine loaded-polymeric microspheres: preparation and physical characteristics. Int J Pharm 175:61–74
Ikenoue T, Akiyoshi M, Fujitani S, Okazaki K, Kondo N, Maki T (1997) Hypoglycemic and insulinotropic effects of a novel oral antidiabetic agent ()-N-(trans-4-sopropylcyclohexanecarbonyl)-d-phenylalanine (A-4166). Br J Pharmacol 120:137–145
Keilson L, Mather S, Walter YH, Subramanian S, Mcleod JF (2000) Determination of nateglinide in human plasma by high-performance liquid chromatography with pre-column derivatization using a coumarin-type fluorescent reagent. J Clin Endocrinol Metab 85:1081
Li S, Lin S, Chien YW, Daggy BP, Mirchandani HL (2001) Statistical optimization of gastric floating system for oral controlled delivery of calcium. AAPS Pharm Sci Tech 2: article 1
Naik JB, Deshmukh RK, Kamle VV (2013) Development of sustained released microparticles of Diclofenac sodium using polymer complex by spray drier. AJPTR 3:892–904
Nateglinide tablets. US FDA label. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021204s014lbl.pdf
Nazzal S, Khan MA (2002) Response surface methodology for the optimization of ubiquinone self-nanoemulsified drug delivery system. AAPS Pharm Sci Tech 3: article 3
Shinkai H, Toi H, Kumashiro I, Seto Y, Fukuma M, Dan K, Toyoshima SN (1988) Acylphenylalanines and related compounds. A new class of oral hypoglycemic agents. J Med Chem 31:2092–2097
Subhedar P, Naik JB, Muley DN (2010) Effect of polymer concentration on sustained release microparticles of metformin hydrochloride prepared by using spray dryer. Polym Plast Eng Technol 49:267–271
Takesada H, Matsuda K, Ohtake R (1996) Structure determination of metabolites isolated from urine and bile after administration of AY4166, a novel d-phenylalanine derivative hypoglycemic agent. Bioorg Med Chem 4:1771–1781
Weaver ML, Orwig BA, Rodriguez LC (2001) Pharmacokinetics and metabolism of nateglinide in humans. Drug Metab Dispos 29:415–421
Ziyaur R, Kanchan K, Khar RK, Mushir A, Charoo NA, Shamsher AA (2006) Characterization of 5-fluorouracil microsphere for colonic delivery. AAPS Pharm Sci Tech 7 (2): E1–E9 (article 47)
Acknowledgement
The authors are grateful to Defense Research and Development Organization, New Delhi, Govt. Of India, for providing financial support, in terms of Extramural Research Project [ERIP/ER/0903820/M/01/1379]. The authors also like to thank University Institute of Chemical Technology, North Maharashtra University, Jalgaon 425 001, Maharashtra, India for providing best facility to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khairnar, G., Mokale, V. & Naik, J. Formulation and development of nateglinide loaded sustained release ethyl cellulose microspheres by O/W solvent emulsification technique. Journal of Pharmaceutical Investigation 44, 411–422 (2014). https://doi.org/10.1007/s40005-014-0118-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-014-0118-3