Abstract
Background
In Germany, the outbreak of the novel pandemic 2009 influenza A(H1N1) virus A(H1N1)pdm09 caused a wave of high activity between November 2009 and January 2011. The aim of this study was to investigate the prevalence of 19 respiratory pathogens in children hospitalized for lower respiratory tract infections during the winter influenza seasons of 2009/2010 and 2010/2011 and to observe a possible impact of influenza A(H1N1)pdm09 on the epidemiology of other epidemic viruses.
Materials and methods
Specimens were nasopharyngeal aspirates which had been collected from children admitted to the participating hospitals in the area of Mainz, Wiesbaden, and Kiel, Germany, with acute community-acquired lower respiratory tract infections. The specimens were subjected to a previously described multiplex reverse transcription PCR assay to detect the following microorganisms: enterovirus, influenza virus types A and B, respiratory syncytial virus (RSV), parainfluenzavirus types 1–4, adenovirus, Mycoplasma pneumoniae, Chlamydophila pneumoniae, rhinovirus, human metapneumovirus (hMPV), coronavirus OC43 and 229E, influenza A(H1N1)pdm09, Bordetella pertussis, Bordetella parapertussis, and Legionella pneumophila.
Results
A total of 3,998 clinical specimens were collected from July 2009 to March 2011, of which 296 were positive for A(H1N1)pdm09. An epidemic of seasonal influenza A or B was not observed in the 2009/2010 season, but a minor epidemic of seasonal influenza B was observed in January/February 2011. Influenza A(H1N1)pdm09 coincided with the absence of the seasonal influenza A of former years. The RSV and hMPV epidemics of 2009/2010 erupted several weeks later than expected based on data collected in the PID-ARI-Network during the past 10 years, whereas in the 2010/2011 influenza season they occurred as expected.
Conclusions
The emergence of the novel influenza A(H1N1)pdm09 virus may have been influenced the epidemiology of other epidemic viruses, such as the RSV and hMPV. No epidemic of seasonal influenza was observed in the 2009/2010 influenza season.
Similar content being viewed by others
Introduction
Respiratory infections occur at a higher frequency in early life than in adulthood [1], with children experiencing approximately five to six infections per year [2, 3]. Influenza activity in Germany has a strong and clear seasonality, with peak epidemic periods occurring during the winter [4].The pandemic novel swine-origin influenza type A virus A(H1N1)pdm09 has disseminated globally after initially being identified in Mexico and the USA in April 2009. This virus appeared in Germany at the end of April 2009 and continued to spread throughout the summer, with a first wave of influenza emerging around July and August 2009, although at low activity. These cases were predominantly infections introduced by travelers returning from countries with a notable prevalence of influenza A(H1N1)pdm09 infections. A few weeks later an increase in the number of indigenous cases was also observed in Germany [5]. At the end of 2009, around week 40, a second increase in epidemic influenza A(H1N1)pdm09 virus activity was noted which reached its peak in week 47, as described previously [6]. This activity of the influenza A(H1N1)pdm09 virus occurred more than 3 months earlier than that normally observed for the seasonal influenza epidemics during past years that typically start around December/January. Altogether, from April 2009 to April 2010, 226,140 confirmed influenza A(H1N1)pdm09 cases were reported to the Robert-Koch-Institute, of which 253 were fatal. Peak activity occurred in November 2009 [6, 7], and by December 2009 the number of new infections had distinctly decreased, possibly due to the vaccination of risk groups with the pandemic vaccine. A second, possibly contributing factor to this decrease in infections may have been the growing awareness of the general population and, accordingly, a more pronounced adherence to personal hygiene, leading to containment of future infections [6].
Despite several reports on the influenza A(H1N1)pdm09 worldwide pandemic, the question of a possible epidemiological shift in the occurrence of competing pathogens has not been addressed. This question can be examined by applying a feasible, sensitive, and specific method for the detection of a broad spectrum of organisms suitable for diagnosing individual cases and epidemiological studies.
In comparison to other influenza infections, the severity and virulence of infections caused by the influenza A(H1N1)pdm09 virus were less prominent, and the majority of cases, including severe cases, were reported in individuals in the age range of 5–24 years [6].
The purpose of this study was to investigate the prevalence and epidemiology of 19 respiratory viruses in infants and children hospitalized for lower respiratory tract infections and to recognize the impact influenza A(H1N1)pdm09 epidemic on the occurrence of other viral pathogens during the winter seasons 2009/2010 and 2010/2011. These were the first seasons with an influenza A(H1N1)pdm09 epidemic since its initial appearance in April 2009 in Germany.
Methods
The multiplex reverse transcription PCR established in our laboratory combined with a microwell hybridization assay (m-RT–PCR–ELISA) is capable of detecting 19 different microorganisms in a single test [8]. This method enables the majority of non-colonizing organisms of the upper respiratory tract to be detected in only one m-RT–PCR procedure. For 14 years, within the research network Pediatric Infectious Diseases Network on Acute Respiratory Tract Infections (PID-ARI.net), our group has been following continuously the epidemiology of non-colonizing bacteria and viruses that cause acute respiratory tract infections in children. This long-term study has enabled us to predict the beginning and activity of respiratory tract epidemics commonly appearing in Germany [9–11].
In order to account to meet the demand for influenza virus analysis during the 2009 epidemic of influenza type A(H1N1)pdm09 virus, we replaced reovirus (Reo) with H1N1 in the assay. Thus, the spectrum of detected microorganisms in this method included: enterovirus (EV), influenza virus type A (IVA) and type B (IVB), respiratory syncytial virus (RSV), parainfluenzavirus types1 (PIV1), 2 (PIV2), 3 (PIV3), and 4 (PIV4), adenovirus (AV), Mycoplasma pneumoniae (Mpn), Chlamydophila pneumoniae (Cpn), rhinovirus (RV), human metapneumovirus (MPV), coronavirus OC43 and 229E (CV), new influenza A(H1N1)pdm09, Bordetella pertussis (Bp) and Bordetella parapertussis (Bpp) and Legionella pneumophila (Lpn). These pathogens do not usually colonize the respiratory tract of humans, but, if present, they are often involved in respiratory disease. Not included was human Bocavirus, a pathogen that has been associated with acute respiratory disease [12].
The specimens for analysis were nasopharyngeal aspirates in NaCl (0.9 %) collected from children admitted to the Department of Pediatrics at the University in Mainz, Wiesbaden and Kiel, Germany, with acute community-acquired lower respiratory tract infections. The specimens were collected following hospitalization, brought directly to the laboratory, and processed immediately (during working hours) or stored at 4 °C (after working hours) until they were processed the next working day. Specimens were split under a laminar airflow. One aliquot of each sample was treated for nucleic acid extraction, and the second was stored at −80 °C. A 200-μl sample of respiratory specimens was subjected to nucleic acid extraction with the High Pure Viral Nucleic Acid kit (Roche Diagnostics, Mannheim, Germany) and eluted into 50 μl of the supplied elution buffer. The eluted DNA was then analyzed using our m-RT–PCR–ELISA system, as described previously [8, 13, 14].
Results
A total of 3,998 clinical specimens were collected from July 2009 to March 2011, of which 296 tested positive for influenza A(H1N1)pdm09 virus. Consequently, influenza A(H1N1)pdm09 virus was the third most detected pathogen between July 2009 and March 2011 (Table 1).
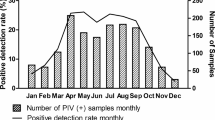
In parallel with the reports of the Robert-Koch-Institute, the influenza A(H1N1)pdm09 epidemic peaked between weeks 43/2009 and 1/2010, with high activity found in week 46/2009. This period of peak activity was preceded by a minor epidemic between weeks 33/2009 and 39/2009 (Fig. 1). During the season of 2010/2011 the influenza A(H1N1)pdm09 epidemic occurred mainly between weeks 2/2011 and 7/2011.
Detection of “top seven” epidemic pathogens in the period between weeks 33/2009 and 26/2010. Incidence of positive influenza A(H1N1) virus detections with a peak in week 46 is given in red. See "Methods" for the definitions of the abbreviations for the pathogens
Prediction versus outcome
Based on reports collected by the PID-ARI.net covering the last 9 years it has been possible to estimate the beginning and activity of respiratory tract epidemics for selected pathogens in Germany [8]. This information has enabled us to predict that the RSV season typically starts in consecutive years (like 2004/2005) quite early and with high activity, whereas the hMPV shows a pronounced and early epidemic in odd years. The yearly seasonal influenza epidemic typically appears around the turn of the year.
RSV
According to this prediction we expected a late start of the RSV epidemic for the season 2009/2010. As expected, the first RSV-positive samples were detected at the end of December 2009. This slightly later detection of RSV may have been due to an impact of the influenza A(H1N1)pdm09 virus, but the displacement was not distinct (Figs. 1, 2). For the following influenza season of 2010/2011 we expected an early RSV epidemic. This was indeed the case, with an RSV epidemic starting in week 40/2010 and enhancing in activity over the following weeks and months with a strong peak in week 1/2011 (Fig. 2).
hMPV
In the season 2009/2010, the hMPV epidemic was expected to start early (September/October 2009) and to be vigorous. However, it started with a delay of several weeks, although the intensity did correspond to our expectations (Figs. 1, 3). A possible explanation for the delay might be that the hMPV and influenza A(H1N1)pdm09 virus were competing for the human host and that the influenza A(H1N1)pdm09 virus was disseminating faster in the population, thereby causing a greater number of infections and thus suppressing the hMPV epidemic for several weeks. In addition, a more pronounced compliance by the general population with personal hygiene measures may have played a role in the containment of hMPV but not in the containment of the more rapidly propagating influenza A(H1N1)pdm09 virus. However, the fact that the endemic rhinoviruses were not suppressed by the influenza A(H1N1)pdm09 virus favors the hypothesis of the hMPV and influenza A(H1N1)pdm09 virus having a more specific impact.
Detection of epidemic pathogens during 1996–2011. Important seasonal pathogens and epidemiology since 1996 based on data in the Pediatric Infectious Diseases Network on Acute Respiratory Tract Infections (PID-ARI.net). Incidence of positive H1N1 detections with a peak in November 2009 and January 2011 is shown in light blue
Influenza A and B
An epidemic of yearly seasonal influenza (A or B) was expected to start in January 2010, similar to almost every other year. However, only sporadic cases were observed, and no epidemic occurred in the season 2009/2010 (Fig. 3). This is in agreement with the data of the influenza sentinel system at the Robert-Koch-Institute, Germany [7]. To which extent a complete suppression by influenza A(H1N1)pdm09 virus had taken place or whether a cross-immunity had built up in the general population which might have been effective against seasonal influenza can only be determined by complex and extensive serological tests which are not feasible. In parallel, the same phenomenon was observed during the season of 2010/2011, when no seasonal influenza A was detected. An influenza B epidemic was detected from January 2011 to March 2011 (Fig. 3).
Double or multiple infections
More double or multiple infections were observed with the influenza A(H1N1)pdm09 virus (7.6 %) during the 2009/2010 season than in former years with other viruses (since 2003; mean 5.2 %). Remarkably, individuals infected with the influenza A(H1N1)pdm09 virus appeared to be more prone to double or multiple infections (13.7 %) than those with the seasonal influenza A or B in epidemics in former years (since 2003; mean 8.5 %). In particular, we detected co-infections with the influenza A(H1N1)pdm09 virus and enteroviruses or rhinoviruses. This might be due to the fact that the influenza A(H1N1)pdm09 epidemic occurred during the autumn, when the incidence of picornaviruses is at its highest.
Discussion
Since its emergence in April 2009, the pandemic A(H1N1)pdm09 virus has disseminated all over the world. On 11 June 2009, the World Health Organization (WHO) raised the pandemic alert level to 6, signifying the first pandemic since 1968 [15]. During the winter season 2009/2010 the new A(H1N1)2009 virus may have altered the epidemiology of other epidemic pathogens. Such interferences are currently the subject of epidemiological research as reliable data and explanations of this phenomenon are currently unavailable.
No epidemic of seasonal influenza was observed in the 2009/2010 winter season, leading to the conclusion that the seasonal influenza was displaced completely by influenza A(H1N1)pdm09. In addition, peak RSV and hMPV activity occurred later in the 2009/2010 winter season, when compared to their seasonal distributions over the past decade, suggesting that the 2009 H1N1 virus may have influenced the epidemiology of these important respiratory viruses. Available data on the innate immune system response to hMPV infections suggest that differences may exist between hMPV and RSV and that hMPV may induce a distinct host response, possibly characterized by potent innate responses to infection [16].
In response to viral infections, plasmacytoid dendritic cells secrete interferon alpha which renders cells refractory to viral infection. Thus, a refractory period develops during influenza infection which protects the cells of the respiratory tract from becoming infected by other respiratory viruses [17]. Respiratory viruses do not bind to the same receptors in the cells of the respiratory system, i.e., human rhinoviruses bind the human intercellular adhesion molecule-I (ICAM-I), whereas influenza viruses bind sialic acid alpha 2–6 [18, 19]. The duration of the refractory period has still to be determined but may persist during virus shedding, i.e., for some weeks [20]. These observations are important because viral interference has an impact on the prediction models for influenza virus dissemination. Such models are an outstanding feature of the rational adoption of resolutions in disease control.
According to the WHO the pandemic influenza A(H1N1)pdm09 virus is expected to take on the behavior of a seasonal influenza virus and continue to circulate [21]. This was the case, based on our observations, during the 2010/2011 season where seasonal influenza A was completely replaced by influenza A(H1N1)pdm09. This finding corresponds to reports from other European countries [22].
In general, pandemic H1N1 incidence should decrease in the longer term because of herd immunity from recent pandemic H1N1 infections, vaccination, and pre-existing cross-protective antibodies in older individuals [23]. In the short term, however, the real situation is different. In the UK, for example, influenza A(H1N1)pdm09 caused more hospital admissions, more critical care admissions, and more deaths in its second year of circulation than in the pandemic year itself due to a variety of reasons, such as a low level of public interest and a lower usage of antiviral drugs [24]. In Spain, significant epidemiological changes and an increased severity of influenza A(H1N1)pdm09 pneumonia were found in the first post-pandemic influenza season [25]. Although the situation in Spain appears to be an exception in comparison to other countries, it helps researchers understand the level of influence the response of local governments has on the spread of a viral infection.
Modeling and predicting the spread of pathogens are important factors when rational decisions are to be made on how to handle epidemics and pandemics. Apart from immunity in the population, factors such as climate, social behavior and possibly co-infections, affect the spread of influenza viruses [26]. Further investigations aimed at elucidating other aspects of such multiple virus interactions, such as the impact of influenza A(H1N1)pdm09 infection on children aged <2 years of age, when there is a high risk of infection by RSV, are ongoing. Finally, pandemic influenza virus may limit the spread of seasonal influenza viruses, as has been reported in the southern hemisphere.
References
Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh Study. JAMA. 1974;227:164–9.
Chonmaitree TK, Revai K, Grady JJ, Clos A, Patel JA, Nair S, Fan J, Henrickson KJ. Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis. 2008;46:815–23.
Grüber C, Keil T, Kulig M, Roll S, Wahn U, Wahn V, MAS-90 Study Group. History of respiratory infections in the first 12 year among children from a birth cohort. Pediatr Allergy Immunol. 2008;19:505–12.
Willers H, Höpken W, Knocke KW. Epidemiologie der Influenza. Med Welt. 1977;28:1595–8.
Surveillance Group for New Influenza A(H1N1). Virus investigation and control in Spain. New influenza A(H1N1) virus infections in Spain, April–May 2009. Euro Surveill. 2009;14:pii=19209.
Krause G, Buchholz U. Epidem Bull 2010–2021 (RKI web site). Available at: http://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2010/Ausgaben/21_10.pdf?__blob=publicationFile.
Buda S, Wilking H, Schweiger B, Buchholz U, Köpke K, Haas W, AGI Study Group. Influenza Wochenbericht. RKI surveillance 2009 (RKI web site). Available at: http://influenza.rki.de/Wochenberichte/2009_2010/2009-51.pdf.
Puppe W, Weigl J, Gröndahl B, Knuf M, Rockahr S, von Bismarck P, Aron G, Niesters HG, Osterhaus AD, Schmitt HJ. Validation of a multiplex reverse transcriptase PCR ELISA for the detection of 19 respiratory tract pathogens. Infection. 2012;. doi:10.1007/s15010-012-0298-6.
Weigl JA, Puppe W, Meyer CU, Berner R, Forster J, Schmitt HJ, Zepp F. Ten years’ experience with year-round active surveillance of up to 19 respiratory pathogens in children. Eur J Pediatr. 2007;166:957–66.
Weigl JA, Puppe W, Meyer CU, Berner R, Forster J, Schmitt HJ, Zepp F. PID-ARI.net—a pediatric infectious diseases network on acute respiratory infections and the added value of a multilevel research network. Klin Padiatr. 2008;220:281–6.
Weigl JA, Puppe W, Belke O, Neusüss J, Bagci F, Schmitt HJ. The descriptive epidemiology of severe lower respiratory tract infections in children in Kiel, Germany. Klin Padiatr. 2005;217:259–67.
Schildgen O, Müller A, Allander T, et al. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin Microbiol Rev. 2008;21:291–304.
Gröndahl B, Puppe W, Hoppe A, Kühne I, Weigl JA, Schmitt HJ. Rapid identification of nine microorganisms causing acute respiratory tract infections by single-tube multiplex reverse transcription-PCR: feasibility study. J Clin Microbiol. 1999;37:1–7.
Puppe W, Weigl JA, Aron G, Gröndahl B, Schmitt HJ, Niesters HG, Groen J. Evaluation of a multiplex reverse transcriptase PCR ELISA for the detection of nine respiratory tract pathogens. J Clin Virol. 2004;30:165–74.
Chan M. World now at the start of 2009 influenza pandemic (WHO website). Available at: http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html.
Schildgen V, van den Hoogen B, Fouchier R, et al. Human Metapneumovirus: lessons learned over the first decade. Clin Microbiol Rev. 2011;24:734–54.
Casalegno JS, Ottmann M, Bouscambert Duchamp M, Escuret V, Billaud G, Frobert E, Morfin F, Lina B. Rhinoviruses delayed the circulation of the pandemic influenza A (H1N1) virus in France. Clin Microbiol Infect. 2009;2010:326–9.
Smith EC, Popa A, Chang A, Masante C, Dutch RE. Viral entry mechanisms: the increasing diversity of paramyxovirus entry. FEBS J. 2009;276:7217–27.
Nicholls J, Chan RW, Russell RJ, Air GM, Peiris JS. Evolving complexities of influenza virus and its receptors. Trend Microbiol. 2008;16:149–57.
Jartti T, Lehtinen P, Vuorinen T, Koskenvuo M, Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol. 2004;72:695–9.
Chan M. H1N1 in post-pandemic period. Media statement (WHO website). Available at: http://www.who.int/mediacentre/news/statements/2010/h1n1_vpc_20100810/en/index.html.
Amato-Gauci A, Zucs P, Snacken R, Ciancio B, Lopez V, Broberg E, Penttinen P, Nicoll A; European Influenza Surveillance Network EISN. Surveillance trends of the 2009 influenza A(H1N1) pandemic in Europe. Euro Surveill 2011;16:pii=19903.
Kok J, Dwyer DE. How common was 2009 pandemic influenza A H1N1? Lancet Infect Dis. 2011;11:423–4.
Mytton OT, Rutter P, Donaldson LJ. Influenza A(H1N1)pdm09 in England, 2009 to 2011: a greater burden of severe illness in the year after the pandemic than in the pandemic year. Euro Surveill 2012;17:pii=20139.
Viasus D, Cordero E, Rodríguez-Baño J, et al. Changes in epidemiology, clinical features and severity of influenza A(H1N1) 2009 pneumonia in the first post-pandemic influenza season. Clin Microbiol Infect. 2012;18:E55–62.
Linde A, Rotzén-Ostlund M, Zweygberg-Wirgart B, et al. Does viral interference affect spread of influenza? Euro Surveill 2009;14:pii=19354.
Acknowledgments
We appreciate the excellent technical assistance of E. Budo-Guetaifi.
Conflict of interest
There is no actual or potential conflict of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gröndahl, B., Ankermann, T., von Bismarck, P. et al. The 2009 pandemic influenza A(H1N1) coincides with changes in the epidemiology of other viral pathogens causing acute respiratory tract infections in children. Infection 42, 303–308 (2014). https://doi.org/10.1007/s15010-013-0545-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-013-0545-5