Abstract
NRF2 is a nuclear transcription factor activated in response to oxidative stress and related with metabolizing of xenotoxic materials and ABC transporter mediated drug resistance. We studied the expression of mRNAs under the siRNA-mediated knockdown of NRF2 and tBHQ-treated condition in AsPC-1 metastatic pancreatic cancer cell line to understand the AsPC-1 specific role(s) of NRF2 and further to investigate the relationship between drug resistance and metastatic plasticity and mobility of AsPc1. Here we show that the genes of aldo–keto reductases, cytochrome P450 family, aldehyde dehydrogenase, thioredoxin reductase, ABC transporter and epoxide hydrolase responsible for drug metabolism or oxidative stress concisely responded to NRF2 stabilization and knockdown of NRF2. In addition the expression of PIR, a candidate of oncogene and KISS1, a suppressor of metastasis were affected by NRF2 stabilization and knockdown. Our result provide comprehensive understanding of NRF2 target genes of drug response, oxidative stress response and metastasis in AsPc-1 metastatic pancreatic cancer cell line.
Similar content being viewed by others
Introduction
Every cell is inevitably exposed to extracellular and intracellular oxidative stress, every moment (Finkel 2011; Ma 2010). The nuclear factor erythroid 2-related factor 2 (NRF2 or NFE2L2) is a master transcription factor that activates a battery of genes which have roles in oxidative stress responses, detoxifications, and drug resistances (Bryan et al. 2013; Ma 2013; Mitsuishi et al. 2012; Niture et al. 2014). NRF2 binds to a DNA element, named antioxidant response element (ARE), in the promoter regions of its target genes to activate transcription of these genes (Nguyen et al. 2003). The target genes of NRF2 includes (a) antioxidant genes such as NAD(P)H dehydrogenase [quinone] 1 (NQO1), heme oxygenase (decycling) 1 (HMOX1), superoxide dismutase [Cu–Zn] (SOD1), and glutamate-cysteine ligase catalytic subunit (GCLC); (b) detoxification genes including glutathione S-transferase A3 (GSTA3) and thioredoxin reductase 1, cytoplasmic (TXNRD1); (c) and drug resistance genes such as ATP-binding cassette sub-family G member 2 (ABCG2) and ATP-binding cassette, sub-family C (CFTR/MRP), member 5 (ABCC5) (Malhotra et al. 2010; Nguyen et al. 2003).
Reactive oxygen species (ROS), which are produced by various exogenous or endogenous sources, are double-edge swords. Under tight cellular control, ROS act as important signaling molecules to regulate diverse cellular functions including transcriptional regulation and signal transduction (Corcoran and Cotter 2013; Finkel 2011; Jennings et al. 2013; Ma 2010; Ray et al. 2012). On the contrary uncontrolled production of ROS causes various human diseases through DNA damage and impaired cellular functions via oxidative stress (Acharya et al. 2010; Caputo et al. 2012; Kakehashi et al. 2013; Kryston et al. 2011; Saeidnia and Abdollahi 2013; Storr et al. 2013). As an ROS sensor, the level of NRF2 is tightly regulated by a set of proteins through proteasome-dependent proteloysis. The well-known negative regulator of NRF2 is the Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1 (KEAP1). KEAP1 binds and destabilized NRF2 through ubiquitin-dependent proteasomal degradation under normal reducing condition (Bryan et al. 2013; Mitsuishi et al. 2012; Niture et al. 2014; Storr et al. 2013). NRF2 stability is also regulated by the CR6-interacting factor 1 (CRIF1) under both reducing and oxidative stress conditions (Kang et al. 2010) and the glycogen synthase kinase 3β (GSK3β)/β-transducin repeat-containing protein (β-TrCP) axis (Chowdhry et al. 2013; Rada et al. 2011; Rada et al. 2012). It has been reported that stability of NRF2 is also regulated by competitive protein–protein interaction to inhibit NRF2-KEAP1 binding by various proteins such as p21 (Chen et al. 2009), the Wilms tumor gene on X chromosome (WTX) (Camp et al. 2012), p62 (Komatsu et al. 2010), the partner and localizer of BRCA2 (PALB2) (Ma et al. 2012), the dipeptidyl peptidase III (DPP3) (Hast et al. 2013), and the breast cancer susceptibility gene 1 (BRCA1) (Gorrini et al. 2013).
NRF2 functions as either a protector against tumorigenesis or oncogene (DeNicola et al. 2011; Kensler and Wakabayashi 2010; Loboda et al. 2008; Muller and Hengstermann 2012). Stability and activity of NRF2 is important in human diseases, especially in cancers. While NRF2 decreases tumor susceptibility in most carcinogenesis models, constitutive activation of NRF2 may enhance tumor cell proliferation and/or confer drug resistance in lung, pancreatic as well as colorectal cancer cells (Arlt et al. 2013; Bryan et al. 2013; Duong et al. 2014b; Homma et al. 2009; Hong et al. 2010; Lister et al. 2011; Mitsuishi et al. 2012; Niture et al. 2014; Singh et al. 2008; Storr et al. 2013; Yamadori et al. 2012). Indeed, NRF2 is up-regulated in many types of tumors through somatic mutations that block KEAP1-dependent regulation of NRF2 stability (Mitsuishi et al. 2012; Niture et al. 2014; Storr et al. 2013). Targeting NRF2 either by RNA interference or by small molecules inhibited tumor growth and increased efficacy of chemotherapy (Singh et al. 2008) or EGF-driven proliferation (Yamadori et al. 2012) in non-small cell lung cancer models and reduced the proliferation and drug-resistance in human lung cancer cells (Homma et al. 2009) or human pancreatic cancer cells (Arlt et al. 2013; Duong et al. 2014b; Hong et al. 2010; Lister et al. 2011). Additionally in primary murine cell models, oncogenes including K-Ras, B-Raf, and Myc increased the transcription of Nrf2 gene to activate antioxidant and detoxification program preferable for oncogenesis (Kang et al. 2014). Under these conditions, genetic targeting of K-RasG12D-driven Nrf2 impaired in vivo tumorigenesis (Kang et al. 2014). Taken together, genome-wide analysis of NRF2-responsive genes in specific cancer types will give insights on the context-dependent roles of NRF2. In this work we delineated NRF2-responsive genes in As-PC1 pancreatic cancer cell lines established from metastatic cancer cell in ascites fluid (Chen et al. 1982).
Materials and methods
Cell culture and reagents
AsPC-1 cells were obtained from the Korean Cell Line Bank (Seoul, Korea) and maintained in RPMI-1640 media (HyClone, Logan, UT) supplemented with 20 % FBS (Invitrogen, Carlsbad, CA) and 100 U/ml penicillin/streptomycin (Welgene, Daegu, Korea). The cells were cultured in a humidified 5 % CO2 incubator at 37 °C. The cell viability and cell counting were assessed by the Luna Automated Cell Counter (Logos Biosystems, Gyunggi-do, Korea). Tert-butylhydroquinone (tBHQ) was purchased from Sigma (St. Louis, MO) and stored at −20 °C dissolved in DMSO with small aliquots.
siRNA transfection
For NRF2 knockdown, exponentially proliferating cells were transfected with synthesized control siRNA (5′-gacgagcggcacgugcacauu-3′) or NRF2 specific siRNA (5′-gaguaugagcuggaaaaacuu-3′) (Hong et al. 2010), both purchased from Bioneer (Daejeon, Korea) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol.
Cell cycle analysis
Cell cycle analysis was carried out by propidium iodide staining and laser detection of FL2 signal using FACSCalibur (BD Science, Franklin Lakes, NJ), and the data were analyzed by CellQuest Pro software (BD Science). After treatment (72 h for siRNA and 16 h for tBHQ treatment respectively), cells were washed with PBS, fixed 70 % ethanol, and stained with propidium iodide solution (20 μg/ml) containing RNaseA (100 μg/ml) after removal of ethanol.
RNA extraction
Total RNA from AsPC-1 cell lines were prepared with the RNeasy Mini kit (Qiagen, Valencia, CA) according to the manufacturer’s protocols. The purity and integrity of RNA sample was evaluated by determining the OD260/230 ratio, 28S/18S ratio, peak pattern and electrophoretic migration patterns on Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA).
Western blot analysis
After 72 h of siRNA treatment or 16 h of tBHQ treatment, AsPC-1 cells were lysed in 10 mM Tris–HCl (pH 7.0), 100 mM NaCl, 1 % triton X-100, 1 mM DTT, 20 μg/ml aprotinin, 2.5 μg/ml leupeptin, and 0.5 mM PMSF. Lysates were resolved on 10 % sodium dodecyl sulfate–polyacrylamide by gel electrophoresis (SDS-PAGE) and transferred onto 0.45 μm pore size Polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA), and immunoblotted with following antibodies: Cyclin B1 antibody (CST#4135, Cell Signaling Technology, Danvers, MA), Cyclin D1 (CST #2922, Cell Signaling Technology), NRF2 (sc-103032, Santa Cruz Biotechnology, Santa Cruz, CA), Erk-1 (sc-94, Santa Cruz Biotechnology), Cyclin A (sc-239, Santa Cruz Biotechnology). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit (sc-2004, Santa Cruz Biotechnology) or anti-mouse antibodies (sc-2005, Santa Cruz Biotechnology) were used as secondary antibodies.
cDNA microarray analysis
The cDNA microarray analysis was carried out with fluorescence labeling of cRNA and hybridization using 4 × 44 K Human whole genome microarray (Agilent technologies, Palo Alto, CA) for tBHQ treated cells. For cDNA microarray analysis of NRF2 siRNA treated cell, Ilumina Biochip system (HT-12) was used. For each microarray three RNA samples of independent experiment were used.
Statistical analysis
Data were analyzed by either Student’s t test (tBHQ treated sample) or LPE test (siRNA treated sample) (Jain et al. 2003) and the results have been expressed p values and mean values.
Results and discussion
The AsPC-1 pancreatic cancer cell line, used in this work had been established from metastatic abdominal ascites fluid cells originated from metastatic pancreatic cancer (Chen et al. 1982). It contains well known mutations of pancreatic cancer including, KRAS (p.G12D), TP53 (p.C135fsP35), SMAD4 (p.R100T), and other mutations common in cancers as well: COL2A1 (c.915 + 3A > G), FBXW7 (p.R465C), HEY1 (p.I178V), KIF5B (p.Q467K), MLL (p.P3536H), RNF43 (p.S720*) (Deer et al. 2010). The relative expression level of NRF2 between various pancreatic cancer cell lines including immortalized human pancreatic ductal epithelial cell lines (HPDE) using GEO2R analysis with pre-deposited microarray data (Thu et al. 2014) at NCBI Gene Expression Ominbus (http://www.ncbi.nlm.nih.gov/geo/geo2r/?acc=GSE40099&platform=GPL6480) is presented in supplementary Fig. 1. NRF2 was reported to be increased in pancreatic cancer cell lines and the nuclear level of NRF2 in AsPC-1 cell line has been reported to be relatively higher than in immortalized pancreatic ductal epithelial cells (Hong et al. 2010; Lister et al. 2011).
Cell cycle analysis of NRF2 siRNA-treated or tBHQ-treated AsPC-1 cell line. a A representative image of FL2 histogram of FACS analysis. The figure in the lower right quadrant is combined FACS analysis images with notion of 2 and 4 N nuclear ploidy. b Immunoblot anaylysis of tBHQ-treated (100 μM 16 h) or siRNA-treated samples (72 h). AsPC-1 cells were seeded in 6-well plates and treated with tBHQ (or DMSO) or NRF2 siRNA (or control siRNA). Cells were harvested and whole cell lysates were prepared, electrophoresed and transferred onto PVDF membranes. Immunoblotting was performed with indicated antibodies and Erk-1 was used as loading control
An antioxidant tBHQ increases the level of NRF2 protein by stabilization and stimulates the expression of oxidative stress metabolizing genes (Hirose et al. 1993; Li et al. 2005). Prior to cDNA microarray we tested whether tBHQ or NRF2 siRNA treatment can change the cell cycle of AsPC-1 cell line. As shown in Fig. 1a no apparent change in cell cycle distribution was observed along with no accumulation of sub G1 population. Immunoblot analysis also revealed that no apparent change of cell cycle marker proteins including cyclin B1 and cyclin D. The level of NRF2 protein was shown to be increased in tBHQ treated cells and decreased in NRF2 siRNA treated sample (Fig. 1b).
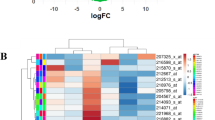
To identify changed genes upon treatment of 100 μM tBHQ, we used the Agilent 44 k whole genome cDNA array chip. We also used the Ilumina HT-12 whole genome cDNA array chip for NRF2 siRNA mediated gene expression analysis. Three independent RNA samples were used in these experiments. After removal of marginal or absent signal spots, 20,312 positive spots were obtained from tBHQ-treated sample and 16,423 positive spots were obtained from NRF2 siRNA-treated sample. Hierarchical cluster image of NRF2 siRNA treatment samples reveals that the gene expression pattern of three siRNA-treated sample and three control siRNA-treated samples are adequately clustered (Fig. 2a). Figure 2b shows the hierarchical cluster image of cDNA microarray of tBHQ-treated sample indicating three independent samples share concordant RNA expression pattern.
Hierarchical cluster image of the gene expression profiles of cDNA microarray analysis. a cDNA array of NRF2 siRNA-treated (2TRE, 3 TRE, 4 TRE) and control siRNA-treated sample (2 Con, 3 Con, 4 Con). Each mRNA sample was labeled and hybridized with cDNA array chip (HT 12, Ilumina) and cluster analysis was carried out. b cDNA array of tBHQ-treated versus DMSO-treated AsPC-1 cells. Two sets of mRNA (tBHQ vs DMSO) with triplicate samples were labeled differently and hybridized. The red color indicated up-regulated genes and the green color indicates down-regulated genes
Further statistic tests after normalization of positive spots provide statistically significant 533 array sets from tBHQ-treated samples (supplementary Table 1) and 189 array sets from NRF2 siRNA-treated samples (supplementary Table 2). Table 1 shows a list of genes which show more than two fold increase of expression (p < 0.05) after treatment of tBHQ (57 genes). Among them AKR1B10, FCER1G, AKR1B1, AKR1B15, AADAC, GRK5, HDAC9, AKR1C1, CYP4F3, CYP4F2, ALDH3A1, FANCD2, TXNRD1 and SLC7A11 are classified as members of drug response genes or oxidative stress response genes according to gene ontology (Table 1). The lists of genes decreased by tBHQ treatment are listed in Table 2. Four genes classified as drug response or oxidative stress response genes were identified: PDE7A, TGM1, CYTH1 and EPS15. The list of top 50 genes which were decreased by NRF2 siRNA treatment are presented in Table 3. The listing is arbitrary but these genes showed more than 40 % reduction in expression. The siRNA mediated knockdown of NRF2 significantly reduced the expression of oxidative stress or drug response genes including, AKR1B10, ALDH1A1, HGD, TFF1, GPX2, ALDH3A1, PPP1R1B, AKR1C4, ABCB6, ABCC3, NFE2L2, EPHX1, ASGR1, SLC2A5, LGALS1 and MTR (Table 3). The expression of NRF2 itself was significantly (p < 0.001, 50 % reduction) decreased by the treatment of siRNA reflecting the reliable quality control of siRNA treatment. On the contrary to NRF2 siRNA treatment the change of NRF2 expression by the tBHQ treatment was not significant (data not shown) since tBHQ stabilized NRF2 protein but had no effect on the mRNA level of NRF2. The array results of increased genes under the NRF2 activated status (tBHQ treatment) and decreased genes by the NRF2 siRNA treatment seem to be coincide. We listed top 50 gene records with increased expression upon NRF2 siRNA treatment in Table 4. Ten genes classified as drug response or oxidative stress response genes were identified as increasing genes in NRF2 siRNA treatment: CD36, ALPP, HLA-B, TGM2, FABP3, CTSH, CYR61, TIMP2, PRNP and NR4A2. We also analyzed metastasis related genes in Tables 1, 2, 3 and 4.
In the cDNA array data of tBHQ-treated cells and NRF2 siRNA-treated samples, total 18 overlapping genes could be obtained with statistical significance (p < 0.05) (Table 5). Unexceptionally 17 genes with increased mRNA expression under the tBHQ treatment showed decreased expression by NRF2 siRNA treatment. The metastasis genes whose relationship with NRF2 was reported previously are as follows: AKR1B10 (Agyeman et al. 2012; Nishinaka et al. 2011), ALDH3A1 (Agyeman et al. 2012), TXNRD1 (Sakurai et al. 2005), AKR1C4 (Ebert et al. 2011), ALDH1A1 (Duong et al. 2014a), PIR (Hubner et al. 2009), GPX2 (Banning et al. 2005), UGDH (Loignon et al. 2009), SRXN1 (Soriano et al. 2008), ME1 (Thimmulappa et al. 2002), ABCB6 (Campbell et al. 2013), EPHX1 (Su et al. 2014), NQO1 (Agyeman et al. 2012; Loignon et al. 2009; Thimmulappa et al. 2002), and ABCC3 (Adachi et al. 2007). Interestingly, we identified three new genes including ALDH3A2, ASPH, and KISS1 as NRF2-responsive genes in this study. To date no study has been reported the relationship of NRF2 with ALDH3A2, ASPH, and KISS1. KISS1 is a protein with 145 amino acid residues and its role is known as an inhibitor of metastasis (Ji et al. 2013). Overexpression KISS1 inhibits metastatic colony formation in ovarian cancer cell lines (Jiang et al. 2005). However, the role of KISS1 in pancreatic cancers has not yet been elucidated. Previously, a report displayed that NRF2 deficient mice showed higher number of pulmonary metastasis than wild-type mice (Satoh et al. 2010). ShRNA mediated knockdown of NRF2 also enhanced cellular plasticity and motility in HepG2 cell (Rachakonda et al. 2010). However, in esophageal squamous cancer cell line NRF2 suppression downregulated the migration and invasion (Shen et al. 2014). Currently, the potential role of NRF2 in regulation of metastasis is under active investigation.
References
Acharya A, Das I, Chandhok D, Saha T (2010) Redox regulation in cancer: a double-edged sword with therapeutic potential. Oxid Med Cell Longev 3:23–34
Adachi T, Nakagawa H, Chung I, Hagiya Y, Hoshijima K, Noguchi N, Kuo MT, Ishikawa T (2007) Nrf2-dependent and -independent induction of ABC transporters ABCC1, ABCC2, and ABCG2 in HepG2 cells under oxidative stress. J Exp Ther Oncol 6:335–348
Agyeman AS, Chaerkady R, Shaw PG, Davidson NE, Visvanathan K, Pandey A, Kensler TW (2012) Transcriptomic and proteomic profiling of KEAP1 disrupted and sulforaphane-treated human breast epithelial cells reveals common expression profiles. Breast Cancer Res Treat 132:175–187
Arlt A, Sebens S, Krebs S, Geismann C, Grossmann M, Kruse ML, Schreiber S, Schafer H (2013) Inhibition of the Nrf2 transcription factor by the alkaloid trigonelline renders pancreatic cancer cells more susceptible to apoptosis through decreased proteasomal gene expression and proteasome activity. Oncogene 32:4825–4835
Banning A, Deubel S, Kluth D, Zhou Z, Brigelius-Flohe R (2005) The GI-GPx gene is a target for Nrf2. Mol Cell Biol 25:4914–4923. doi:10.1128/MCB.25.12.4914-4923.2005
Bryan HK, Olayanju A, Goldring CE, Park BK (2013) The Nrf2 cell defence pathway: keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol 85:705–717
Camp ND, James RG, Dawson DW, Yan F, Davison JM, Houck SA, Tang X, Zheng N, Major MB, Moon RT (2012) Wilms tumor gene on X chromosome (WTX) inhibits degradation of NRF2 protein through competitive binding to KEAP1 protein. J Biol Chem 287:6539–6550
Campbell MR, Karaca M, Adamski KN, Chorley BN, Wang X, Bell DA (2013) Novel hematopoietic target genes in the NRF2-mediated transcriptional pathway. Oxid Med Cell Longev 2013:120305
Caputo F, Vegliante R, Ghibelli L (2012) Redox modulation of the DNA damage response. Biochem Pharmacol 84:1292–1306
Chen WH, Horoszewicz JS, Leong SS, Shimano T, Penetrante R, Sanders WH, Berjian R, Douglass HO, Martin EW, Chu TM (1982) Human pancreatic adenocarcinoma: in vitro and in vivo morphology of a new tumor line established from ascites. In Vitro Cell Dev Biol Plant 18:24–34
Chen W, Sun Z, Wang XJ, Jiang T, Huang Z, Fang D, Zhang DD (2009) Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol Cell 34:663–673
Chowdhry S, Zhang Y, McMahon M, Sutherland C, Cuadrado A, Hayes JD (2013) Nrf2 is controlled by two distinct beta-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene 32:3765–3781
Corcoran A, Cotter TG (2013) Redox regulation of protein kinases. FEBS J 280:1944–1965
Deer EL, Gonzalez-Hernandez J, Coursen JD, Shea JE, Ngatia J, Scaife CL, Firpo MA, Mulvihill SJ (2010) Phenotype and genotype of pancreatic cancer cell lines. Pancreas 39:425–435
DeNicola GM et al (2011) Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475:106–109
Duong HQ, Yi YW, Kang HJ, Bae I, Jang YJ, Kwak SJ, Seong YS (2014a) Combination of dasatinib and gemcitabine reduces the ALDH1A1 expression and the proliferation of gemcitabine-resistant pancreatic cancer MIA PaCa-2 cells. Int J Oncol 44:2132–2138
Duong HQ, Yi YW, Kang HJ, Hong YB, Tang W, Wang A, Seong YS, Bae I (2014b) Inhibition of NRF2 by PIK-75 augments sensitivity of pancreatic cancer cells to gemcitabine. Int J Oncol 44:959–969
Ebert B, Kisiela M, Wsol V, Maser E (2011) Proteasome inhibitors MG-132 and bortezomib induce AKR1C1, AKR1C3, AKR1B1, and AKR1B10 in human colon cancer cell lines SW-480 and HT-29. Chem Biol Interact 191:239–249
Finkel T (2011) Signal transduction by reactive oxygen species. J Cell Biol 194:7–15
Gorrini C, Baniasadi PS, Harris IS, Silvester J, Inoue S, Snow B, Joshi PA, Wakeham A, Molyneux SD, Martin B, Bouwman P, Cescon DW, Elia AJ, Winterton-Perks Z, Cruickshank J, Brenner D, Tseng A, Musgrave M, Berman HK, Khokha R, Jonkers J, Mak TW, Gauthier ML (2013) BRCA1 interacts with Nrf2 to regulate antioxidant signaling and cell survival. J Exp Med 210:1529–1544
Hast BE, Goldfarb D, Mulvaney KM, Hast MA, Siesser PF, Yan F, Hayes DN, Major MB (2013) Proteomic analysis of ubiquitin ligase KEAP1 reveals associated proteins that inhibit NRF2 ubiquitination. Cancer Res 73:2199–2210
Hirose M, Yada H, Hakoi K, Takahashi S, Ito N (1993) Modification of carcinogenesis by alpha-tocopherol, t-butylhydroquinone, propyl gallate and butylated hydroxytoluene in a rat multi-organ carcinogenesis model. Carcinogenesis 14:2359–2364
Homma S, Ishii Y, Morishima Y, Yamadori T, Matsuno Y, Haraguchi N, Kikuchi N, Satoh H, Sakamoto T, Hizawa N, Itoh K, Yamamoto M (2009) Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin Cancer Res 15:3423–3432
Hong YB, Kang HJ, Kwon SY, Kim HJ, Kwon KY, Cho CH, Lee JM, Kallakury BV, Bae I (2010) Nuclear factor (erythroid-derived 2)-like 2 regulates drug resistance in pancreatic cancer cells. Pancreas 39:463–472
Hubner RH, Schwartz JD, De Bishnu P, Ferris B, Omberg L, Mezey JG, Hackett NR, Crystal RG (2009) Coordinate control of expression of Nrf2-modulated genes in the human small airway epithelium is highly responsive to cigarette smoking. Mol Med 15:203–219
Jain N, Thatte J, Braciale T, Ley K, O’Connell M, Lee JK (2003) Local-pooled-error test for identifying differentially expressed genes with a small number of replicated microarrays. Bioinformatics 19:1945–1951
Jennings P, Limonciel A, Felice L, Leonard MO (2013) An overview of transcriptional regulation in response to toxicological insult. Arch Toxicol 87:49–72
Ji K, Ye L, Mason MD, Jiang WG (2013) The Kiss-1/Kiss-1R complex as a negative regulator of cell motility and cancer metastasis (Review). Int J Mol Med 32:747–754
Jiang Y, Berk M, Singh LS, Tan H, Yin L, Powell CT, Xu Y (2005) KiSS1 suppresses metastasis in human ovarian cancer via inhibition of protein kinase C alpha. Clin Exp Metastasis 22:369–376. doi:10.1007/s10585-005-8186-4
Kakehashi A, Wei M, Fukushima S, Wanibuchi H (2013) Oxidative stress in the carcinogenicity of chemical carcinogens. Cancers 5:1332–1354
Kang HJ, Hong YB, Kim HJ, Bae I (2010) CR6-interacting factor 1 (CRIF1) regulates NF-E2-related factor 2 (NRF2) protein stability by proteasome-mediated degradation. J Biol Chem 285:21258–21268
Kang KA, Piao MJ, Kim KC, Kang HK, Chang WY, Park IC, Keum YS, Surh YJ, Hyun JW (2014) Epigenetic modification of Nrf2 in 5-fluorouracil-resistant colon cancer cells: involvement of TET-dependent DNA demethylation. Cell Death Dis 5:e1183. doi:10.1038/cddis.2014.149
Kensler TW, Wakabayashi N (2010) Nrf2: friend or foe for chemoprevention? Carcinogenesis 31:90–99
Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M (2010) The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 12:213–223
Kryston TB, Georgiev AB, Pissis P, Georgakilas AG (2011) Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res 711:193–201
Li J, Spletter ML, Johnson JA (2005) Dissecting tBHQ induced ARE-driven gene expression through long and short oligonucleotide arrays. Physiol Genomics 21:43–58
Lister A, Nedjadi T, Kitteringham NR, Campbell F, Costello E, Lloyd B, Copple IM, Williams S, Owen A, Neoptolemos JP, Goldring CE, Park BK (2011) Nrf2 is overexpressed in pancreatic cancer: implications for cell proliferation and therapy. Mol Cancer 10:37
Loboda A, Was H, Jozkowicz A, Dulak J (2008) Janus face of Nrf2-HO-1 axis in cancer–friend in chemoprevention, foe in anticancer therapy. Lung Cancer 60:1–3
Loignon M, Miao W, Hu L, Bier A, Bismar TA, Scrivens PJ, Mann K, Basik M, Bouchard A, Fiset PO, Batist Z, Batist G (2009) Cul3 overexpression depletes Nrf2 in breast cancer and is associated with sensitivity to carcinogens, to oxidative stress, and to chemotherapy. Mol Cancer Ther 8:2432–2440
Ma Q (2010) Transcriptional responses to oxidative stress: pathological and toxicological implications. Pharmacol Ther 125:376–393
Ma Q (2013) Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53:401–426
Ma J, Cai H, Wu T, Sobhian B, Huo Y, Alcivar A, Mehta M, Cheung KL, Ganesan S, Kong AN, Zhang DD, Xia B (2012) PALB2 interacts with KEAP1 to promote NRF2 nuclear accumulation and function. Mol Cell Biol 32:1506–1517
Malhotra D, Portales-Casamar E, Singh A, Srivastava S, Arenillas D, Happel C, Shyr C, Wakabayashi N, Kensler TW, Wasserman WW, Biswal S (2010) Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucl Acids Res 38:5718–5734
Mitsuishi Y, Motohashi H, Yamamoto M (2012) The Keap1-Nrf2 system in cancers: stress response and anabolic metabolism. Front Oncol 2:200
Muller T, Hengstermann A (2012) Nrf2: friend and foe in preventing cigarette smoking-dependent lung disease. Chem Res Toxicol 25:1805–1824
Nguyen T, Sherratt PJ, Pickett CB (2003) Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol 43:233–260
Nishinaka T, Miura T, Okumura M, Nakao F, Nakamura H, Terada T (2011) Regulation of aldo-keto reductase AKR1B10 gene expression: involvement of transcription factor Nrf2. Chem Biol Interact 191:185–191
Niture SK, Khatri R, Jaiswal AK (2014) Regulation of Nrf2-an update. Free Radic Biol Med 66:36–44
Rachakonda G, Sekhar KR, Jowhar D, Samson PC, Wikswo JP, Beauchamp RD, Datta PK, Freeman ML (2010) Increased cell migration and plasticity in Nrf2-deficient cancer cell lines. Oncogene 29:3703–3714
Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A (2011) SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol 31:1121–1133
Rada P, Rojo AI, Evrard-Todeschi N, Innamorato NG, Cotte A, Jaworski T, Tobon-Velasco JC, Devijver H, Garcia-Mayoral MF, Van Leuven F, Hayes JD, Bertho G, Cuadrado A (2012) Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/beta-TrCP axis. Mol Cell Biol 32:3486–3499
Ray PD, Huang BW, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24:981–990
Saeidnia S, Abdollahi M (2013) Antioxidants: friends or foe in prevention or treatment of cancer: the debate of the century. Toxicol Appl Pharmacol 271:49–63
Sakurai A, Nishimoto M, Himeno S, Imura N, Tsujimoto M, Kunimoto M, Hara S (2005) Transcriptional regulation of thioredoxin reductase 1 expression by cadmium in vascular endothelial cells: role of NF-E2-related factor-2. J Cell Physiol 203:529–537
Satoh H, Moriguchi T, Taguchi K, Takai J, Maher JM, Suzuki T, Winnard P T Jr, Raman V, Ebina M, Nukiwa T, Yamamoto M (2010) Nrf2-deficiency creates a responsive microenvironment for metastasis to the lung. Carcinogenesis 31:1833–1843
Shen H, Yang Y, Xia S, Rao B, Zhang J, Wang J (2014) Blockage of Nrf2 suppresses the migration and invasion of esophageal squamous cell carcinoma cells in hypoxic microenvironment. Dis Esophagus 27:685–692
Singh A, Boldin-Adamsky S, Thimmulappa RK, Rath SK, Ashush H, Coulter J, Blackford A, Goodman SN, Bunz F, Watson WH, Gabrielson E, Feinstein E, Biswal S (2008) RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res 68:7975–7984
Soriano FX, Leveille F, Papadia S, Higgins LG, Varley J, Baxter P, Hayes JD, Hardingham GE (2008) Induction of sulfiredoxin expression and reduction of peroxiredoxin hyperoxidation by the neuroprotective Nrf2 activator 3H-1,2-dithiole-3-thione. J Neurochem 107:533–543
Storr SJ, Woolston CM, Zhang Y, Martin SG (2013) Redox environment, free radical, and oxidative DNA damage. Antioxid Redox Signal 18:2399–2408
Su S, Yang X, Omiecinski CJ (2014) Intronic DNA elements regulate Nrf2 chemical responsiveness of the human microsomal epoxide hydrolase gene (EPHX1) through a far upstream alternative promoter. Biochim Biophys Acta 1839:493–505
Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S (2002) Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res 62:5196–5203
Thu KL, Radulovich N, Becker-Santos DD, Pikor LA, Pusic A, Lockwood WW, Lam WL, Tsao MS (2014) SOX15 is a candidate tumor suppressor in pancreatic cancer with a potential role in Wnt/beta-catenin signaling. Oncogene 33:279–288
Yamadori T, Ishii Y, Homma S, Morishima Y, Kurishima K, Itoh K, Yamamoto M, Minami Y, Noguchi M, Hizawa N (2012) Molecular mechanisms for the regulation of Nrf2-mediated cell proliferation in non-small-cell lung cancers. Oncogene 31:4768–4777
Conflict of interest
Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Yi, Y.W., Oh, S. Comparative analysis of NRF2-responsive gene expression in AcPC-1 pancreatic cancer cell line. Genes Genom 37, 97–109 (2015). https://doi.org/10.1007/s13258-014-0253-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-014-0253-2