Abstract
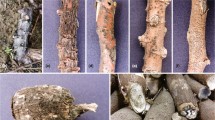
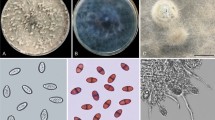
The global expansion of cultivation areas of Jatropha has contributed to the emergence of various diseases. Currently in Brazil, the occurrence of a new disease has been reported that not only reduces the productivity but also causes the death of Jatropha. This disease is associated with collar and root rot of plants. From morphological and phylogenetic studies (based on Internal Transcribed Spacers, β-tubulin and Translation Elongation Factor 1-α sequences), nine species of Botryosphaeriaceae were identified. These species include Lasiodiplodia egyptiacae, L. pseudotheobromae, L. theobromae, Macrophomina phaseolina, Neoscytalidium hyalinum and four Lasiodiplodia spp. that are proposed as new species (L. euphorbicola, L. jatrophicola, L.macrospora and L. subglobosa). All the species in this study, except M. phaseolina, are pathogenic. The results show that root rot of physic nut plants is caused by complex pathogens. This study provides new information for future studies of disease management, quarantine programs and, especially, the development of resistant varieties for collar and root rot disease in J. curcas.








Similar content being viewed by others
References
Abbas SQ, Sutton BC, Ghaffar A, Abbas A (2004) Reassessment of Sphaeropsis undulata Berk. & Curt. Pak J Bot 36:209–218
Abdollahzadeh J, Jvadi A, Mohammadi-Goltapeh E, Zare R, Phillips AJL (2010) Phylogeny and morphology of four new species of Lasiodiplodia from Iran. Persoonia 25:1–10
Akintayo ET (2004) Characteristics and composition of Parkia biglobosa and Jatropha curcas oils and cakes. Bioresour Technol 92:307–310
Alves A, Crous PW, Correia A, Phillips AJL (2008) Morphological and molecular data reveal cryptic species in Lasiodiplodia theobromae. Fungal Divers 28:1–13
Begoude BAD, Slippers B, Wingfield MJ, Roux J (2010) Botryosphaeriaceae associated with Terminalia catappa in Cameroon, South Africa and Madagascar. Mycol Prog 9:101–123
Burgess TI, Barber PA, Mohali S, Pegg G, de Beer W, Wingfield MJ (2006) Three new Lasiodiplodia spp. from the tropics, recognized based on DNA sequence comparisons and morphology. Mycologia 98:423–435
Cai L, Udayanga D, Manamgoda DS, Maharachchikumbura SSN, McKenzie EHC, Guo LD, Liu XZ, Bahkali A, Hyde KD (2011) The need to carry out re-inventory of plant pathogenic fungi. Trop Plant Pathol 36:205–213
Carbone I, Anderson JB, Kohn LM (1999) A method for designing primer sets for the speciation studies in filamentous ascomycetes. Mycologia 91:553–556
Cardoso JE, Wilkinson MJ (2008) Desenvolvimento e caracterização de marcadores microsatélites para o fungo Lasiodiplodia theobromae. Summa Phytopathol 34:55–57
Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Phillips AJL, Alves A, Burgess T, Barber P, Groenewald JZ (2006) Phylogenetic lineages in the Botryosphaeriaceae. Stud Mycol 55:235–253
Damm U, Crous PW, Fourie PH (2007) Botryosphaeriaceae as potential pathogens of Prunus in South Africa, with descriptions of Diplodia africana and Lasiodiplodia plurivora sp. nov. Mycologia 99:664–680
Dhingra OD, Sinclair JB (1978) Biology and pathology of Macrophomina phaseolina. Imprensa Universitária, Universidade Federal de Viçosa, Viçosa, Brasil, 166 pp
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Etayo J, Diederich P (1996) Lichenicolous fungi from the western Pyrenees, France and Spain. II. More deuteromycetes. Mycotaxon 60:419–420
Fischer IH, Almeida AM, Garcia MJM, Bertani RMA, Bueno CJ (2008) First report of Lasiodiplodia theobromae on Asclepias physocarpa in Brazil. Australas Plant Dis Notes 3:116–117
Fujinawa MF, Pontes NC, Souza ESC, Goes A, Vale HMM (2012) First report of Lasiodiplodia theobromae causing stem rot disease of begonia (Begonia x elatior hort.) in Brazil. Australas Plant Dis Notes 7:163–166
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330
Halfeld-Vieira BA, Nechet KL (2005) Queda de frutos em coqueiro causada por Lasiodiplodia theobromae em Roraima. Fitopatol Bras 30:203
Hall T (2012) BioEdit v7.0.9: Biological sequence alignment editor for Win95/98/2K/XP/7. Available at http://www.mbio.ncsu.edu/bioedit/bioedit.html Acessed 15 July 2012
Hyde KD, Abd-Elsalam K, Cai L (2010) Morphology: still essential in a molecular world. Mycotaxon 114:439–451
Ismail AM, Cirvilleri G, Polizzi G, Crous PW, Groenewald JZ, Lombard L (2012) Lasiodiplodia species associated with dieback disease of mango (Mangifera indica) in Egypt. Australas Plant Pathol 41:649–660
Jacobs K, Bergdahl DR, Wingfield MJ, Halik S, Seifert KA, Bright DE, Wingfield BD (2004) Leptographium wingfieldii introduced into North America and found associated with exotic Tomicus piniperda and native bark beetles. Mycol Res 108:411–418
Jami F, Slippers B, Wingfield MJ, Gryzenhout M (2012) Five new species of the Botryosphaeriaceae from Acacia karroo in South Africa. Cryptog Mycolog 33:245–266
Latha P, Prakasam V (2012) Molecular variability in Lasiodiplodia theobromae causing collar and root rot in physic nut (Jatropha curcas). J Mycol Plant Pathol 42:356–360
Latha P, Prakasam V, Kamalakannan A, Gopalakrishnan C, Raguchander T, Paramathma M, Samiyappan R (2009) First report of Lasiodiplodia theobromae (Pat.) Griffon & Maubl causing root rot and collar rot disease of physic nut (Jatropha curcas L.) in India. Australas Plant Dis Notes 4:19–20
Liu JK, Phokamsak R, Doilom M, Wikee S, Li YM, Ariyawansha H, Boonmee S, Chomnunti P, Dai DQ, Bhat JD, Romero AI, Zhuang WY, Monkai J, Jones EBG, Chukeatirote E, Ko TWK, Zhao YC, Wang Y, Hyde KD (2012) Towards a natural classification of Botryosphaeriales. Fungal Divers 57:149–210
Machado AR, Pereira OL (2012) Major diseases of the biofuel plant, physic nut(Jatropha curcas). In: Fang Z (ed) Biodiesel: Feedstocks, production and applications. Intech, Croatia, pp 59–75
Machado AR, Pinho DB, Dutra DC, Pereira OL (2012) Collar and root rot caused by Neoscytalidium dimidiatum in the biofuel plant Jatropha curcas. Plant Dis 96:1697
Marques MW, Lima NB, Morais JRMA, Barbosa MAG, Souza BO, Michereff SJ, Phillips AJL, Camara MPS (2013a) Species of Lasiodiplodia associated with mango in Brazil. Fungal Divers 61:181–193
Marques MW, Lima NB, Morais MA Jr, Michereff SJ, Phillips AJL, Câmara MPS (2013b) Botryosphaeria, Neofusicoccum, Neoscytalidium and Pseudofusicoccum species associated with mango in Brazil. Fungal Divers 61:195–208
Mendes MAS, Urben AF (2012) Fungos relatados em plantas no Brasil, Laboratório de Quarentena Vegetal. Brasília, DF: Embrapa Recursos Genéticos e Biotecnologia. Available at http://pragawall.cenargen.embrapa.br/aiqweb/michtml/fgbanco01.asp Acessed 10 July 2012
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES science gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE). New Orleans LA, USA. pp. 1−8
Paim ECA, Silveira AJ, Bezerra JL, Martins ED, Luz MN, Sacramento CK (2012) Etiologia do declínio de mangostanzeiros no sul da Bahia. Rev Bras Frutic 34:1074–1083
Patel DS, Patel SI, Patel RL (2008) A new report on root rot of Jatropha curcas caused by Macrophomina phaseolina from Gujarat, India. J Mycol Plant Pathol 38:356–357
Pavlic D, Slippers B, Coutinho TA, Gryzenhout M, Wingfield MJ (2004) Lasiodiplodia gonubiensis sp. nov., a new Botryosphaeria anamorph from native Syzygium cordatum in South Africa. Stud Mycol 50:313–322
Pavlic D, Wingfield MJ, Barber P, Slippers B, Hardy GESJ, Burgess TI (2008) Seven new species of the Botryosphaeriaceae from baobab and other native trees in Western Australia. Mycologia 100:851–866
Pavlic D, Slippers B, Coutinho TA, Wingfield MJ (2009) Molecular and phenotypic characterization of three phylogenetic species discovered within the Neofusicoccum parvum/N. ribis complex. Mycologia 101:636–647
Pereira AL, Silva GS, Ribeiro VQ (2006) Caracterização fisiológica, cultural e patogênica de diferentes isolados de Lasiodiplodia theobromae. Fitopatol Bras 31:572–578
Pereira OL, Dutra DC, Dias LAS (2009) Lasiodiplodia theobromae is the causal agent of a damaging root and collar rot disease on the biofuel plant Jatropha curcas in Brazil. Australas Plant Dis Notes 4:120–123
Petrak F (1923) Mycologische Notizen V. No. 200. Über die Pseudosphaeriaceen v.H. und ihre Bedeutung für die spezielle Systematik der Pyrenomyzeten. Ann Mycol 21:30–69
Phillips AJL, Alves A, Correia A, Luque J (2005) Two new species of Botryosphaeria with brown, 1-septate ascospores and Dothiorella anamorphs. Mycologia 97:513–529
Phillips AJL, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald JZ, Crous PW (2013) The Botryosphaeriaceae: genera and species known from culture. Stud Mycol 76:51–167
Pinho DB, Firmino AL, Pereira OL, Ferreira Junior WG (2012) An efficient protocol for DNA extraction from Meliolales and the description of Meliola centellae sp. nov. Mycotaxon 122:333–345
Polizzi G, Aiello D, Vitale A (2009) First report of shoot blight, canker, and gummosis caused by Neoscytalidium dimidiatum on citrus in Italy. Plant Dis 93:1215
Posada D, Buckley TR (2004) Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst Biol 53:793–808
Pramanik K (2003) Properties and use of Jatropha curcas oil and diesel fuel blends in compression ignition engine. Renew Energ 28:239–248
Rambaut A (2009) FigTree 1.2.2. Available at http://tree.bio.ed.ac.uk/software/figtree/. Accessed 15 January 2010
Rannala B, Yang Z (1996) Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J Mol Evol 43:304–311
Ray JD, Burgess T, Lanoiselet VM (2010) First record of Neoscytalidium dimidiatum and N. novaehollandiae on Mangifera indica and N. dimidiatum on Ficus carica in Australia. Australas Plant Dis Notes 5:48–50
Ronquist F, Heulsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Saccardo PA (1899) Syll Fung 14:938
Saccardo PA (1913) Syll Fung 22:1012
Saccardo PA (1915) Fungi ex insula Melita (Malta) lecti a Doct. Caruana-Gatto et Doct. G. Borg annis MCMXIII et MCMIV. Nuovo Giorn Bot Ital 22:61
Saturnino HM, Pacheco DD, Kakida J, Tominaga N, Gonçalves NP (2005) Cultura do pinhão-manso (Jatropha curcas L.). Informe Agropecuário 26:44–78
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci 109:6241–6246
Slippers B, Wingfield MJ (2007) Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biol Rev 21:90–106
Sutton BC (1980) The Coelomycetes: Fungi imperfecti with acervuli, pycnidia and stromata. Commonwealth Mycological Institute, Kew
Sydow H (1924) Beschreibungen neuer südafrikanischer Pilze IV. Ann Mycol 22:430–431
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: Molecular evolutionary genetics analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol 28:2731–2739
Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, Hibbett DS, Fisher MC (2000) Phylogenetic species recognition and species concepts in fungi. Fungal Genet Biol 31:21–32
Úrbez-Torres JR, Leavitt GM, Voegel TM, Gubler WD (2006) Identification and distribution of Botryosphaeria spp. associated with grapevine cankers in California. Plant Dis 90:1490–1503
Urbez-Torres JR, Peduto F, Striegler RK, Urrea-Romero KE, Rupe JC, Cartwright RD, Gubler WD (2012) Characterization of fungal pathogens associated with grapevine trunk diseases in Arkansas and Missouri. Fungal Divers 52:169–189
White TJ, Bruns T, Lee S, Taylor J (1990) Amplifcation and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Acknowledgments
This work forms part of a research project submitted as an M.Sc. dissertation to the Departamento de Fitopatologia/Universidade Federal de Viçosa by A. R. Machado. The authors thank Fundação de Amparo a Pesquisa do Estado de Minas Gerais –FAPEMIG for the financial support of the work (CAG-APQ-00987-11) and Conselho Nacional de Desenvolvimento Científico e Tecnológico –CNPq for authors fellowships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Machado, A.R., Pinho, D.B. & Pereira, O.L. Phylogeny, identification and pathogenicity of the Botryosphaeriaceae associated with collar and root rot of the biofuel plant Jatropha curcas in Brazil, with a description of new species of Lasiodiplodia . Fungal Diversity 67, 231–247 (2014). https://doi.org/10.1007/s13225-013-0274-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-013-0274-1