Abstract
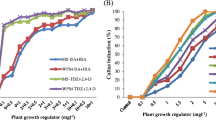
Factors affecting in vitro propagation and microtuberization were evaluated for Gloriosa superba L., an endangered ornamental cum medicinal plant having limited reproductive capacity. Surface sterilization of tuber explants with 0.1% mercuric chloride (HgCl2) for 5 min eliminated the contamination effectively with highest survival rate. Among the various combinations used, Murashige and Skoog (MS) medium with 2.0 mg L−1 6-benzylaminopurine (BAP) + 0.5 mg L−1 α-naphthalene acetic acid (NAA) containing 3% sucrose with 16-h photoperiod exhibited the greatest in vitro tuberization (3.2) with the highest shoot regeneration frequency (90%). The longest tuber regeneration occurred on MS media containing 4% sucrose. Transfer of in vitro-regenerated shoots to half-strength MS medium with 1.0 mg L−1 indole-3-butyric acid (IBA) + 0.5 mg L−1 NAA showed maximum root induction (66.6%). The in vitro-grown plantlets were successfully acclimatized and transplanted to sterilized soil and sand mixture (3:1) in the glasshouse with 70% survival. The colchicine content was determined in the tubers of ex vitro plants by HPLC using the same retention time (1.5 min) as that of the standard colchicine. This revealed that the micropropagation protocol developed by us for rapid mass production could be used as raw material for colchicine extraction and provides a basis for germplasm conservation and genetic improvement of G. superba.
Similar content being viewed by others
References
Ade R, Rai MK. 2009. Review: Current advances in Gloriosa superba L. Biodiversitas 10: 210–214
Aksenova NP, Konstamtinova TN, Golyanovskaya SS, Kossmann J, Willmitzer L, Romanov. 2000. In vitro microtuberization in Potato. Russ. J. Plant Physiol. 133: 23–27
Alali F, Tawaha K, Qasaymch RM. 2004. Determination of colchicine in Colchicum steveni and C. hierosolymitanum (Colchicaceae): Comparison between two analytical methods. Phytochem. Anal. 15: 27–29
Amoroso EC. 1935. Colchicine and tumour growth. Nature 135: 266–267
Ang BH, Chan LK. 2003. Micropropagation of Spilanthes acmella L., a bio-insecticide plant, through proliferation of multiple shoots. J. Appl. Hortic. 5: 65–68
Anonymous. 1956. The Wealth of India: a dictionary of Indian raw material and industrial products. CSIR, Delhi. 4: 139–140
Behera KK, Sahoo S, Prusti A. 2009. Regeneration of plantlet of water yam (Dioscorea oppositifoliaa L.) through in vitro culture from nodal segments. Not. Bot. Hort. Agrobot. Cluj. 37: 94–102
Bernier G, Pe’rilleux C. 2005. A physiological overview of the genetics of flowering time control. Plant Biotechnol. J. 3: 3–16
Bhojwani SS, Razdan MK. 2005. Production of secondary metabolites. In Plant Tissue Culture: Theory and Practice, Rev. eds, Amsterdam, Elsevier, pp 537–562
Charles G, Rossignol L, Rossignol M. 1992. Environmental effects on potato plants in vitro. J. Plant Physiol. 139: 708–713
Chawla HS. 2003. Plant Biotechnology: A Practical Approach. Science Publishers Inc., Enfield (NH), USA, ISBN:157808296X, pp 51–55
Chen FQ, Fu Y, Wang DL, Gao X, Wang L. 2007. The effect of plant growth regulators and sucrose on the micropropagation and microtuberization of Dioscorea nipponica Makino. J. Plant Growth Regul. 26: 38–45
Fernie AR, Willmitzer L. 2001. Molecular and biochemical triggers of potato tuber development. Plant Physiol. 127: 1459–1465
Forsyth C, Van Staden J. 1984. Tuberization of Dioscorea bulbifera stems nodes in culture. J. Plant Physiol. 115: 79–83
Ghosh B, Mukherjee S, Jha TB, Jha S. 2002. Enhanced colchicine production in root cultures of Gloriosa superba by direct and indirect precursors of the biosynthetic path ways. Biotechnol. Lett. 24: 231–234
Ghosh S, Ghosh B, Jha S. 2006. Aluminium chloride enhances colchicine production in root cultures of Gloriosa superba. Biotechnol. Lett. 28: 497–503
Goyal D, Bhadauria S. 2006. In vitro propagation of Ceropegia bulbosa using nodal segments. Ind. J. Biotech. 5: 565–568
Grison C. 1991. Influence des facteurs d’environnement sur le cycle ve’ge’tatif de la pomme de terre. La pomme de terre française 462: 7–15
Hara M, Tanaka S, Tabata M. 1994. Induction of a specific methyltransferase activity regulating berberine biosynthesis by cytokinin in Thalictrum minus cell cultures. Phytochemistry 36: 327–332
Hassan AKMS, Roy SK. 2005. Micropropagation of Gloriosa superba L. through high frequency shoot proliferation. Plant Tiss. Cult. 15: 67–74
Jana S, Shekhawat GS. 2011a. Critical review on medicinally potent plant species: Gloriosa superba. Fitoterapia 82: 293–301
Jana S, Shekhawat GS. 2011b. Plant growth regulators, adenine sulfate and carbohydrates regulate organogenesis and in vitro flowering of Anethum graveolens. Acta Physiol. Plant. 33: 305–311
Jo EA, Tewari RK, Hahn EJ, Paek KY. 2009. In vitro sucrose concentration affects growth and acclimatization of Alocasia amazonica plantlets. Plant Cell Tiss. Organ Cult. 96: 307–315
Kala C, Farooquee N, Dhar U. 2004. Prioritization of medicinal plants on the basis of available knowledge, existing practices and use value status in Uttaranchal, India. Biodivers. Conserv. 13: 453–469
Komalavalli N, Rao MV. 2000. In vitro micropropagation of Gymnema sylvestre — A multipurpose medicinal plant. Plant Cell Tiss. Org. Cult. 61: 97–105
Krause J. 1986. Production of Gloriosa tubers from seeds. Acta Hortic. 177: 353–360
Kumar S, Mangal M, Dhawan AK, Singh N. 2011. Assessment of genetic fidelity of micropropagated plants of Simmondsia chinensis (Link) Schneider using RAPD and ISSR markers. Acta Physiol. Plant. 33: 2541–2545
Lawrence CH, Barker WG. 1963. A study of tuberization in the potato, Solanum tuberosum. Amer. Potato J. 40: 349–356
Maiti CK, Sen S, Paul AK, Acharya K. 2007. First report of leaf blight disease of Gloriosa superba L. caused by Alternaria alternata (Fr.) Keissler. Ind. J. Plant Pathol. 73: 377–378
Mantell SH, Hugo SA. 1989. Effect of photoperiod, mineral medium strength, ionic ammonium and cytokinin on root, shoot and microtuber development in shoot cultures of D. alata and D. bulbifera yam. Plant Cell Tiss. Organ Cult. 16: 23–27
Medina RD, Flachsland EA, Gonzalez AM, Terada G, Faloci MM, Mroginski LA. 2009. In vitro tuberization and plant regeneration from multimodal segment culture of Habenaria bractescens Lindl., an Argentinean wetland orchid. Plant Cell Tiss. Organ Cult. 97: 91–101
Murashige T, Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497
Ohyama K. 1970. Tissue culture in mulberry tree. Jpn. Agric. Res. Quart. 5: 30–34
Omokolo ND, Boudjeko T, Tsafack TJJ. 2003. In vitro tuberization of Xanthosoma sagittifolium (L.) Schott: Effects of phytohormones, sucrose, nitrogen and photoperiod. Sci. Hortic. 98: 337–345
Omokolo ND, Tsala NG, Kanmegne G, Balange AP. 1995. Production of multiple shoots, callus, plant regeneration and tuberization in Xanthosoma sagittifolium cultured in vitro. C R Acad. Sci. 318: 773–778
Ovono PO, Kevers C, Dommes J. 2007. Axillary proliferation and tuberization of Dioscorea cayenensis-D. rotundata complex. Plant Cell Tiss. Org. Cult. 91: 107–114
Ovono PO, Kevers C, Dommes J. 2009. Effects of reducing sugar concentration on in vitro tuber formation and sprouting in yam (Dioscorea cayenensis-D. rotundata complex). Plant Cell Tiss. Organ Cult. 99: 55–59
Pan RC. 2001. Phytophysiology 4th edn. Higher Education Press, Beijing, China, pp 176–186
Pelacho AM, Mingo-Castel AM. 1991. Effects of photoperiod on kinetin-induced tuberization of isolated potato stolons cultured in vitro. Am. Potato J. 68: 533–541
Perl A, Aviv D, Willmitzer L, Galun E. 1991. In vitro tuberization in transgenic potatoes harboring-glucuronidase linked to a patatin promoter: effects of sucrose levels and photoperiods. Plant Sci. 73: 87–95
Pierik RLM. 1967. Factors affecting the induction and initiation of flower buds in vitro in plant species with a cold requirement for flowering. In G Bernier, eds, Cellular and Molecular Aspects of Floral Induction, Longman Grp Ltd., London, pp 408–4115
Poornima GN, Rai VR. 2007. In vitro propagation of wild yams, Dioscorea oppositifolia (Linn) and Dioscorea pentaphylla (Linn). Afr. J. Biotechnol. 6: 2348–2352
Rai VR. 2002. Rapid clonal propagation of Nothapodytes foetida (Weight) Sleumer — a threatened medicinal tree. In Vitro Cell Dev. Biol. Plant 38: 347–351
Robins RJ, Parr AJ, Payne J, Walton NJ, Rhodes MJC. 1990. Factors regulating tropane-alkaloid production in a trans formed root culture of a Datura candida X D. aurea hybrid. Planta 181: 414–422
Sahoo Y, Chand PK. 1998. Micropropagation of Vitex negundo L., a woody aromatic medicinal shrub, through high frequency axillary shoot proliferation. Plant Cell Rep. 18: 301–307
Sarkar D. 2008. The signal transduction pathways controlling in planta tuberization in potato: An emerging synthesis. Plant Cell Rep. 27: 1–8
Satyavati GV, Raina MK, Sharma M. 1976. Medicinal Plants of India. ICMR, Delhi, Vol.1, pp 433–434
Seabrook JEA, Coleman S, Levy D. 1993. Effect of photoperiod on in vitro tuberization of potato (Solanum tuberosum L.). Plant Cell Tiss. Org. Cult. 34: 43–51
Sivakumar G, Krishnamurthy KV. 2000. Micropropagation of Gloriosa superba L-an endangered species of Asia and Africa. Curr. Sci. 78: 31–32
Sivakumar G, Krishnamurthy KV. 2002. Gloriosa superba L. — a very useful medicinal plant. In VK Singh, JN Govil, S Hashmi, G Singh, eds, Recent Progress in Medicinal Plants, Vol. 7, Ethnomedicine and Pharmacognosy, Part II. Series Sci. Tech. Pub., Texas, USA, pp 465–82
Sivakumar G, Krishnamurthy KV. 2004. In vitro organogenetic responses of Gloriosa superba. Russ. J. Plant Physiol. 51: 790–798
Tiwari KN, Sharma NC, Tiwari V, Singh BD. 2000. Micropropagation of Centella asiatica (L.), a valuable medicinal herb. Plant Cell Tiss. Organ Cult. 63: 179–185
Vaillant V, Bade P, Constant C. 2005. Photoperiod affects the growth and development of yam plantlets obtained by in vitro propagation. Biol. Plant. 49: 355–359
Yadav K, Singh N. 2011. Effect of seed harvesting season and sterilization treatments on germination and In vitro propagation of Albizia lebbeck (L.) Benth. Analele din Oradea - Fascicula Biologie 18: 151–156
Yadav K, Singh N. 2012. Factors influencing in vitro plant regeneration of Liquorice (Glycyrrhiza glabra L.). Iranian J. Biotechnol. 10: 161–167
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yadav, K., Aggarwal, A. & Singh, N. Actions for ex situ conservation of Gloriosa superba L. - an endangered ornamental cum medicinal plant. J. Crop Sci. Biotechnol. 15, 297–303 (2012). https://doi.org/10.1007/s12892-012-0045-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12892-012-0045-7