Abstract
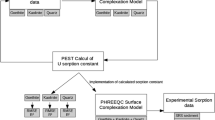
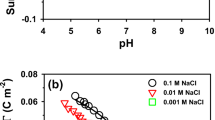
Surface complexation models (SCMs) are widely utilized as a tool to study the mobility of radionuclide to the environment. In this study, two SCMs, electrostatic (ES) and non-electrostatic (NES) models are employed in order to simulate the sorption behavior of U(VI) on quartz in the absence of Mg, Ca, and Sr and ES model in the presence of alkaline earth metals. The surface reaction constants (log K) for ES and NES models were fitted to experimental data by coupling PEST with PHREEQC. The SCM prediction of U(VI) sorption on quartz in the absence of alkaline earth metals is in good agreement with the experimental data in the pH range 6.5–9. The estimated log K values are sensitive to both ES and NES model calculations. In U(VI)-Mg-quartz, U(VI)-Ca-quartz, and U(VI)-Sr-quartz systems, only the ES model shows the general trend of the experimental data. The estimated log K values are sensitive with respect to the surface reactions. Hence, the ES model is more suitable for model calculations of the U(VI)-Mg-quartz, U(VI)-Ca-quartz, and U(VI)-Sr-quartz systems.



Similar content being viewed by others
References
Appelo CAJ, Van der Weiden MJJ, Tournassat C, Charlet L (2002) Surface complexation of ferrous iron and carbonate on ferrihydrite and the mobilization of arsenic. Environ Sci Technol 36(14):3096–3103
Aytas SM, Akyil S, Eral M (2004) Adsorption and thermodynamic behavior of uranium on natural zeolite. J Radioanal Nucl Chem 260(1):119–125
Bachmaf S, Merkel BJ (2011) Sorption of Uranium(VI) at the clay mineral-water interface. Environ Earth Sci 63(5):925–934
Barnett MO, Jardine PM, Brooks SC (2002) U(VI) adsorption to heterogeneous subsurface media: application of a surface complexation model. Environ Sci Technol 36(5):937–942
Benedikt G (2007) 797 VA Computrace–voltammetric trace determination of Uranium(VI) in drinking and mineral water. Metrohm Information Issue 2/2007. Metrohm Ltd.,CH-9101 Herisau, Switzerland, p 36
Bernhard G, Geipel G, Brendler V, Nitsche H (1996) Speciation of uranium in seepage waters of a mine tailing pile studied by time-resolved laser-induced fluorescence spectroscopy (TRLFS). Radiochim Acta 74:87–91
Bernhard G, Geipel G, Reich T, Brendler V, Amayri S, Nitsche H (2001) Uranyl(VI) carbonate complex formation: validation of the Ca2UO2(CO3)3 (aq.) species. Radiochim Acta 89(8):511–518
Borgnino L, Pauli C, Depetris P (2012) Arsenate adsorption at the sediment-water interface: sorption experiments and modelling. Environ Earth Sci 65(2):441–451
Camacho LM, Deng SG, Parra RR (2010) Uranium removal from groundwater by natural clinoptilolite zeolite: effects of pH and initial feed concentration. J Hazard Mater 175(1–3):393–398
Campos M, Azevedo H, Nascimento M, Roque Cu, Rodgher S (2011) Environmental assessment of water from a uranium mine (Caldas, Minas Gerais State, Brazil) in a decommissioning operation. Environ Earth Sci 62(4):857–863
Chandrajith R, Seneviratna S, Wickramaarachchi K, Attanayake T, Aturaliya TNC, Dissanayake CB (2010) Natural radionuclides and trace elements in rice field soils in relation to fertilizer application: study of a chronic kidney disease area in Sri Lanka. Environ Earth Sci 60(1):193–201
Comarmond MJ, Payne TE, Harrison JJ, Thiruvoth S, Wong HK, Aughterson RD, Lumpkin GR, Muller K, Foerstendorf H (2011) Uranium sorption on various forms of titanium dioxide—influence of surface area, surface charge, and impurities. Environ Sci Technol 45(13):5536–5542
Curtis GP, Davis JA, Naftz DL (2006) Simulation of reactive transport of Uranium(VI) in groundwater with variable chemical conditions. Water Res Res 42(4). doi:10.1029/2005WR003979
Davis JA, Meece DE, Kohler M, Curtis GP (2004) Approaches to surface complexation modeling of Uranium(VI) adsorption on aquifer sediments. Geochim Cosmochim Acta 68(18):3621–3641
Doherty J (2006) PEST, Model-Independent Parameter Estimation, User Manual, 5th edn. Watermark Numerical Computing, Brisbane
Dong WM, Brooks SC (2006) Determination of the formation constants of ternary complexes of uranyl and carbonate with alkaline earth metals (Mg2+, Ca2+, Sr2+, and Ba2+) using anion exchange method. Environ Sci Technol 40(15):4689–4695
Dong WM, Brooks SC (2008) Formation of aqueous MgUO2(CO3) 2−3 complex and uranium anion exchange mechanism onto an exchange resin. Environ Sci Technol 42(6):1979–1983
Du Q, Sun Z, Forsling W, Tang H (1997) Adsorption of copper at aqueous illite surfaces. J Colloid Interface Sci 187(1):232
Dzombak DA, Morel FM (1990) Surface complexation modeling: hydrous ferric oxide. John Wiley & Sons, New York
Ekberg C, Ödegaard-Jensen A, Meinrath G (2002) LJUNGSKILE 1.0: A computer program for investigation of uncertainties in chemical speciation. ISRNSKI-R-03/03-SE
Froideval A, Del Nero M, Gaillard C, Barillon R, Rossini I, Hazemann JL (2006) Uranyl sorption species at low coverage on Al-hydroxide: TRLFS and XAFS studies. Geochim Cosmochim Acta 70(21):5270–5284
Geipel G, Amayri S, Bernhard G (2008) Mixed complexes of alkaline earth uranyl carbonates: a laser-induced time-resolved fluorescence spectroscopic study. Spectrochim Acta A Mol Biomol Spectrosc 71(1):53–58
Gomes MEP, Neves LJPF, Coelho F, Carvalho A, Sousa M, Pereira AJSC (2011) Geochemistry of granites and metasediments of the urban area of Vila Real (northern Portugal) and correlative radon risk. Environ Earth Sci 64(2):497–502
Grenthe I, Lagerman B (1991) Studies on metal carbonate equilibria. 22. A coulometric study of the Uranium(VI)-carbonate system, the composition of the mixed hydroxide carbonate species. Acta Chem Scand 45(2):122–128
Grenthe I, Fuger J, Konings R, Lemire RJ, Muller AB, Wanner J (2007) The Chemical Thermodynamics of Uranium. Elsevier, New York
Guo ZJ, Li Y, Wu WS (2009) Sorption of U(VI) on goethite: effects of pH, ionic strength, phosphate, carbonate and fulvic acid. Appl Radiat Isot 67(6):996–1000
Herbelin AL, Westall JC (1999) FITEQL 4.0: A computer programme for determination of chemical equilibrium constants from experimental data. Report 99-01. Corvallis, Oregon: Department of Chemistry, Oregon State University
Hsi CKD, Langmuir D (1985) Adsorption of uranyl onto ferric oxyhydroxides: application of the surface complexation site-binding model. Geochim Cosmochim Acta 49(9):1931–1941
Huber F, Lützenkirchen J (2009) Uranyl retention on quartz: new experimental data and blind prediction using an existing surface complexation model. Aquat Geochem 15(3):443–456
Hull LC, Schafer AL (2008) Accelerated transport of Sr-90 following a release of high ionic strength solution in vadose zone sediments. J Contam Hydrol 97(3–4):135–157
IAEA (2003) Radiation protection and the management of radioactive waste in the oil and gas industry. Safety Report Series No 34. International Atomic Energy Agency
Illingworth JA (1981) A common source of error in pH measurements. Biochem J 195(1):259–262
Kacmaz H, Eran Nakoman M (2009) Hydrochemical characteristics of shallow groundwater in aquifer containing uranyl phosphate minerals, in the Koprubasi (Manisa) area, Turkey. Environ Earth Sci 59(2):449–457
Kalmykov SN, Choppin GR (2000) Mixed Ca2+/UO2 2+/CO3 2− complex formation at different ionic strengths. Radiochim Acta 88(9–11):603–606
Krepelova A, Sachs S, Bernhard G (2006) Uranium(VI) sorption onto kaolinite in the presence and absence of humic acid. Radiochim Acta 94(12):825–833
Linhoff B, Bennett P, Puntsag T, Gerel O (2011) Geochemical evolution of uraniferous soda lakes in Eastern Mongolia. Environ Earth Sci 62(1):171–183
Meinrath G, Merkel B, Ödegaard-Jensen A, Ekberg C (2004) Sorption of iron on surfaces: modelling, data evaluation, and measurement uncertainty. Acta Hydrochim Hydrobiol 32(2):154
Merkel BJ (2011) Thermodynamic Data Dilemma. In: Merkel BJ, Schipek M (eds) The New Uranium Mining Boom: Challenge and lessons learned. Springer, Berlin Heidelberg, pp 627–633. doi:10.1007/978-3-642-22122-4
Merkel BJ, Hasche-Berger A (2006) Uranium in the environment—mining impact and consequences. Uranium mining and hydrogeology. Springer, Berlin
Merkel BJ, Hasche-Berger A (2008) Uranium, Mining and Hydrogeology. Fifth International Conference Uranium Mining and Hydrogeology (UMH V). Springer, Berlin
Merkel BJ, Nair S (2011) Impact of speciation and Sorption on migration of uranium in groundwater. In: Paul M (ed) Nachhaltigkeit und Langzeitaspekte bei der Sanierung von Uranbergbau und Aufbereitungsstandorten. Proceedings des Internationalen Bergbausymposiums WISSYM_2011. Wismut GmbH, Chemnitz, Germany, pp 269–274
Merkel BJ, Planer-Friedrich B, Wolkersdorfer C (2002) Uranium in the Aquatic Environment. International Conference Uranium Mining and Hydrogeology III and the International Mine Water Association Symposium. Springer, Berlin
Missana T, Garcia-Gutierrez M, Maffiotte C (2003) Experimental and modeling study of the Uranium(VI) sorption on goethite. J Colloid Interface Sci 260(2):291–301
Nair S, Merkel BJ (2011) Impact of alkaline earth metals on aqueous speciation of Uranium(VI) and sorption on quartz. Aquat Geochem 17(3):209–219
Nitzsche O, Meinrath G, Merkel B (2000) Database uncertainty as a limiting factor in reactive transport prognosis. J Contam Hydrol 44(3–4):223
Ödegaard-Jensen A, Ekberg C, Meinrath G (2004) LJUNGSKILE: a program for assessing uncertainties in speciation calculations. Talanta 63(4):907
Pabalan RT, Turner DR (1996) Uranium(6+) sorption on montmorillonite: experimental and surface complexation modeling study. Aquat Geochem 2(3):203–226
Papini MP, Kahie YD, Troia B, Majone M (1999) Adsorption of lead at variable pH onto a natural porous medium: modeling of batch and column experiments. Environ Sci Technol 33(24):4457–4464
Parkhurst DL, Appelo CA (1999) User’s Guide to PHREEQC (version 2). A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculation. USGS, Water Resources Investigation Report pp 99–4259
Prikryl JD, Jain A, Turner DR, Pabalan RT (2001) Uranium(VI) sorption behavior on silicate mineral mixtures. J Contam Hydrol 47(2–4):241–253
Ricka A, Kuchovsky T, Sracek O, Zeman J (2010) Determination of potential mine water discharge zones in crystalline rocks at Rozna, Czech Republic. Environ Earth Sci 60(6):1201–1213
Sherman DM, Peacock CL, Hubbard CG (2008) Surface complexation of U(VI) on goethite (alpha-FeOOH). Geochim Cosmochim Acta 72(2):298–310
Stamberg K, Venkatesan KA, Rao PRV (2003) Surface complexation modeling of uranyl ion sorption on mesoporous silica. Coll Surf A Physicochem Eng Aspects 221(1–3):149–162
Sulekha Rao N, Sengupta D, Guin R, Saha SK (2009) Natural radioactivity measurements in beach sand along southern coast of Orissa, eastern India. Environ Earth Sci 59(3):593–601
Tang YZ, Reeder RJ (2009) Uranyl and arsenate co-sorption on aluminum oxide surface. Geochim Cosmochim Acta 73(10):2727–2743
Turner GD, Zachara JM, McKinley JP, Smith SC (1996) Surface-charge properties and UO2 2+ adsorption of a subsurface smectite. Geochim Cosmochim Acta 60(18):3399–3414
Waite TD, Davis JA, Payne TE, Waychunas GA, Xu N (1994) Uranium(VI) adsorption to ferrihydrite: application of a surface complexation model. Geochim Cosmochim Acta 58(24):5465–5478
Waite TD, Davis JA, Fenton BR, Payne TE (2000) Approaches to modelling Uranium(VI) adsorption on natural mineral assemblages. Radiochim Acta 88(9–11):687–693
Zheng ZP, Tokunaga TK, Wan JM (2003) Influence of calcium carbonate on U(VI) sorption to soils. Environ Sci Technol 37(24):5603–5608
Zhijun G, Zhaoyun Y, Zuyi T (2004) Sorption of uranyl ions on TiO2: effects of contact time, ionic strength, concentration and humic substance. J Radioanal Nucl Chem 261(1):157–162
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nair, S., Karimzadeh, L. & Merkel, B.J. Surface complexation modeling of Uranium(VI) sorption on quartz in the presence and absence of alkaline earth metals. Environ Earth Sci 71, 1737–1745 (2014). https://doi.org/10.1007/s12665-013-2579-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2579-5