Abstract
Purpose
We sought to develop a temperature-based respiratory instrument to measure respiration noninvasively outside critical care settings.
Method
Respiratory temperature profiles were recorded using a temperature-based noninvasive instrument comprised of three rapid responding medical-grade thermistors—two in close proximity to the mouth/nose (sensors) and one remote to the airway (reference). The effect of the gas flow rate on the amplitude of the tracings was determined. The temperature-based instrument, the Linshom Respiratory Monitoring Device (LRMD) was mounted to a face mask and positioned on a mannequin face. Respiratory rates of 5-40 breaths·min−1 were then delivered to the mannequin face in random order using artificial bellows (IngMar Lung Model). Data from the sensors were collected and compared with the bellows rates using least squares linear regression and coefficient of determination. The investigators breathed at fixed rates of 0-60 breaths·min−1 in synchrony with a metronome as their respiratory temperature profiles were recorded from sensors mounted to either a face mask or nasal prongs. The recordings were compared with a contemporaneously recorded sidestream capnogram from a CARESCAPE GEB450 Monitor. The extracted respiratory rates from the LRMD tracings and capnograms were compared using linear regression with a coefficient of determination and a Bland-Altman plot.
Results
The amplitude of the sensor tracings was independent of the oxygen flow rate. Respiratory rates from the new temperature-based sensor were synchronous and correlated identically with both the artificial bellows (r2 = 0.9997) and the capnometer mounted to both the face mask and nasal prongs (r2 = 0.99; bias = −0.17; 95% confidence interval, −2.15 to 1.8).
Conclusions
Respiratory rates using the LRMD, a novel temperature-based respiratory instrument, were consistent with those using capnometry.
Résumé
Objectif
Nous avons tenté de mettre au point un instrument respiratoire se fondant sur la température afin de mesurer la respiration de façon non invasive en dehors des unités de soins critiques.
Méthode
Les profils de température respiratoire ont été enregistrés à l’aide d’un instrument non invasif se fondant sur la température et composé de trois thermistances de qualité médicale à réponse rapide – deux à proximité de la bouche et du nez (capteurs) et un troisième à l’écart des voies aériennes (référence). L’effet du débit gazeux sur l’amplitude des tracés a été déterminé. L’instrument fondé sur la température, nommément le dispositif de monitorage respiratoire Linshom (LRMD), a été fixé à un masque facial et positionné sur le visage d’un mannequin. Des fréquences respiratoires de 5-40 respirations·min−1 ont ensuite été livrées au visage du mannequin dans un ordre aléatoire à l’aide de soufflets artificiels (modèle de poumon IngMar). Les données des capteurs ont été colligées et comparées aux fréquences des soufflets à l’aide d’une méthode de régression linéaire des moindres carrés et d’un coefficient de détermination. Les chercheurs ont respiré à des fréquences fixes de 0-60 respirations·min−1 en synchronie avec un métronome pendant que leurs profils de température respiratoire étaient enregistrés par des capteurs fixés à un masque facial ou à des canules nasales. Les enregistrements ont été comparés à un tracé de capnogramme latéral enregistré simultanément par un moniteur CARESCAPE GEB450. Les fréquences respiratoires extraites des tracés du LRMD et des capnogrammes ont été comparées à l’aide d’une méthode de régression linéaire avec un coefficient de détermination et un graphique de Bland-Altman.
Résultats
L’amplitude des tracés des capteurs était indépendante du débit d’oxygène. Les fréquences respiratoires du nouveau capteur basé sur la température étaient synchrones et identiquement corrélées aux soufflets artificiels (r2 = 0,9997) et au capnomètre fixé au masque facial et aux canules nasales (r2 = 0,99; biais = −0,17; intervalle de confiance 95 %, −2,15 à 1,8).
Conclusion
Les fréquences respiratoires mesurées à l’aide du LRMD, un nouvel instrument respiratoire fondé sur la température, étaient cohérentes à celles mesurées par capnométrie.
Similar content being viewed by others
Outside critical care settings,1-3 there is a paucity of inexpensive and portable monitors that can deliver accurate measurements, monitor respiratory rate and, in particular, detect apnea in advance of desaturation. In many centres, pulse oximetry is used as a surrogate monitor for respiration, although the sensitivity of this monitor to detect apnea is attenuated when oxygen is administered.4 Other monitors, such as capnography and plethysmography, for tracking respiration during transport and monitoring sedation on the ward have their limitations (e.g., cost, size, weight, and reliability) which preclude their routine use.5-7 We identified the need for a new monitor based on simple physiological principles that would track respiration in advance of apnea and address the above shortcomings of the current monitors.
Previously, investigators attempted to monitor indices of respiration by placing a thermistor adjacent to the airway and measuring the changes in the temperature of the breath during the respiratory cycle.8 These detectors did not achieve any measure of clinical success, however, partly because they had slow response times and could not distinguish between the temperature of the exhaled breath and that within the microenvironment of the face mask. The former problem was remedied by the inclusion of medical-grade rapid responding thermistors, but the latter problem persisted. We determined that we could address the latter problem and increase the signal-to-noise ratio of temperature within the face mask by implementing the Peltier-based microbalance in the temperature controller. To address this challenge, we sought to develop a temperature-based respiratory instrument that could reliably and consistently measure the respiratory rate in humans.
Methods
In accordance with the conditions for acceptance by the Journal during its editorial process, this investigation was submitted post hoc to the Institutional Review Board (IRB) at the State University of New York, Buffalo. The IRB determined that this research did not involve human subjects and that approval was not required (dated March 31, 2016). A temperature-based noninvasive instrument, the Linshom Respiratory Monitoring Device (LRMD), was developed to measure respiration. The instrument is comprised of three rapid responding medical-grade thermistors (1.5-mm diameter) with a unique control-loop algorithm executed by a microcontroller.Footnote 1 The two primary thermistors (sensors) are positioned near the mouth and/or nose within a disposable oxygen face mask (Hudson RCI Ref 1041, USA) (Fig. 1) or nasal prongs (Fig. 2) to measure the temperature during respiration. The solitary secondary (reference) thermistor is positioned outside the face mask to measure and adjust for the ambient temperature. The thermistor signals are converted from analog to digital signals using analog-to-digital converters and displayed. The sensors placed in the vicinity of the nose and/or mouth respond precisely by keeping the thermistor in thermal balance with a thermoelectric cooler (TEC) utilizing the Peltier effect within a control-loop process. The TEC selects a temperature setpoint based on ambient conditions and continuously operates to hold the sensor’s temperature stable at that setpoint. As respiration disrupts the thermal balance, the control loop generates a feedback signal (i.e., an accurate signature representation of the breath) which captures the subtle detail of the breath cycle. After each breath, the control loop returns the sensor to thermal balance with smooth precision ready for the next breath.
When the investigators (J.L., R.F., J.M.) self-applied the face masks, data were acquired from the LRMD sensors mounted in the masks, and the extracted data were stored on a laptop using a custom program.
To determine the effect of the oxygen flow rate (2, 4, 6, or 10 L·min−1) on the readings of the LRMD sensor, the investigators breathed 12-30 breaths·min−1 through the oxygen face mask at each of the four oxygen flow rates while the LRMD responses were recorded electronically. The amplitude of the tracings from the nadir of the signal at end-inspiration until the peak at end-exhalation was recorded for each breath at the respective gas flow rate.
A bench test was designed to determine whether this novel sensor could track respiration delivered by calibrated mechanical bellows (IngMar Medical Adult/Pediatric Demonstration Lung Model; IngMar Medical, Pittsburgh, PA, USA). An oxygen face mask fitted with a LRMD sensor was placed over the mannequin’s mouth. The bellows delivered breaths to the mannequin’s mouth via a single tube at respiratory rates of 5-40 breaths·min−1 (with corresponding lung volumes of 120-300 mL·breath−1) in a randomized sequence (using www.random.org). The gas flow cooled the sensor during each breath, and when the breath ended, the TEC automatically returned the thermistor to its setpoint, creating a sinusoidal breathing pattern. Data were collected from the sensor for 30-60 sec at a time depending on the respiratory rate of the bellows. The respiratory rates measured from the tracings detected by the sensor were plotted against those set on the bellows and analyzed using linear regression and the coefficient of determination (r2) using Prism 6.0d software (GraphPad, La Jolla, CA, USA).
Respiratory rate was evaluated while one of the investigators breathed oxygen through an oxygen face mask fitted with a LRMD sensor and operational sidestream capnography. The LRMD sensor was mounted across from the mouth/nose in the mask while data were collected. The end-tidal carbon dioxide tension was measured via nasal prongs and a CARESCAPE GEB450 Monitor (GE Healthcare, Chicago, IL, USA, hereafter referred to as GEB450). The investigator breathed at constant rates of ~7.5-60 breaths·min−1 for two-minute periods in synchrony with a metronome. The sinusoidal tracings from the LRMD sensor and the GEB450 were compared after three manipulations were applied to the LRMD data. Differences in the latency between the two instruments were corrected by digitizing the upstrokes of the initial breath from each monitor and synchronizing them to begin at time 0. These differences were attributed in part to the capacity of the LRMD sensor to detect breaths more rapidly than the capnogram. To ensure a stable breathing pattern, the breath-to-breath interval was determined after at least the third breath in each sequence. Differences in the amplitude of the tracings between the two instruments were adjusted by first normalizing the data from the LRMD sensor by amending each data point by the offset between the minimum value and 0 and then converting the GEB450 values to SI units. Differences in the rate of data acquisition were adjusted by halving the speed of the tracings from the LRMD sensor and then superimposing the tracings from the two instruments. A sample tracing was extracted to illustrate the congruity of the respirations detected by the LRMD and the capnogram. The derived respiratory rates from the two instruments were plotted using linear regression and r2.
The LRMD sensor, with dimensions of 25 mm × 20 mm × 15 mm (length × width × height) and a weight of 10 g, was adapted to nasal prongs (Fig. 2). To evaluate simultaneous recordings of the nasal LRMD tracing and nasal capnometry (GEB450), one investigator breathed 20-40 breaths·min−1 in synchrony with a metronome in random order (predetermined using www.random.org), and the signals were recorded simultaneously. The derived respiratory rates from the two instruments were plotted using a Bland-Altman plot.
To demonstrate that the LRMD sensor responds rapidly to apneas, one investigator breathed through an oxygen face mask until a stable respiratory pattern was detected. The respiratory rate was detected using nasal capnometry and the LRMD monitor mounted to the nasal prongs. Periodic breath holds (apneas) were then performed to demonstrate whether LRMD and the capnometer detected the apneas.
Statistical analysis
The respiratory rates with each oxygen flow rate were compared using one-way analysis of variance. The closeness of fit between the programmed bellows respiratory rate or the GEB450 and that determined by LRMD was compared using least squares regression analysis and the coefficient of variation (r2). A Bland-Altman plot was constructed to show the average respiratory rate from the nasal LRMD and GEB450 capnometer sensors vs the difference in the respiratory rates between the two sensors. All reported P values are two sided.
Results
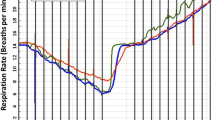
The data from the LRMD sensor were unaffected by oxygen flow rates of 2-10 L·min−1 (P = 0.19) (Fig. 3). Every breath was detected. The variability in the LRMD readings at each flow rate was attributed to differences in tidal volume between breaths.
LRMD sensor readings were recorded while one of the investigators breathed at typical resting tidal volumes through a disposable oxygen face mask. The differences in the readings were not statistically significant at oxygen flow rates of 2-10 L·min−1 (see text). The box and whisker plot displays the five number summary for each oxygen flow rate, median [25th and 75th percentiles], and the minimum and maximum values. n = the number of repetitions performed
Respiratory rates measured by the LRMD sensor correlated identically (r2 = 0.9997) with those delivered by the artificial bellows (Fig. 4).
Respiratory signals from the LRMD sensor in the face mask and GEB450 were synchronous and congruous as depicted in the sample tracing shown in Fig. 5. Each breath detected by the LRMD sensor corresponded one-to-one to the respirations detected by GEB450. The LRMD sensor detected every breath that was detected by the GEB450. Although the shape and maximum end-tidal pCO2 of the GEB450 tracings were fairly consistent (i.e., a constant end-tidal pCO2), the amplitude of the LRMD tracings varied by up to 50% (Fig. 5). The latter variability was attributed to variations in the relative tidal volume.Footnote 2 Respiratory rates determined by the LRMD monitor correlated one-to-one with those from the GEB450 (r2 = 0.99) (Fig. 6).
Respiration was recorded simultaneously using the LRMD sensor mounted to a disposable oxygen face mask (open black circles and the black solid line) and the GEB450 using nasal prongs (solid magenta line). The LRMD units are temperature differences (ΔFo) (right Y-axis) and the capnograph is the end-tidal carbon dioxide concentration (kPa) (left Y-axis). The LRMD measurements shown are the differences in temperature between the LRMD measurement and ambient temperature, each increased by 3.156 to ensure a value of 0 at end-inspiration. The respirations from the LRMD tracings are synchronous and congruent with those displayed by the GEB450. The time units (X-axis) shown are 1,000 units per 1.6 min
With the LRMD monitor mounted to a nasal cannula, a Bland-Altman plot of the average respiratory rates detected by the LRMD and GEB450 monitors yielded a bias of −0.17 with a 95% confidence interval of −2.15 to 1.8 (Fig. 7).
Bland-Altman plot of the difference in the respiratory rates (RR) between the LRMD nasal sensor and the nasal capnometer (Y-axis) vs the average RR of the LRMD nasal sensor and the nasal capnometer (X-axis). Respiratory rates measured by the two sensors correlated closely, with a bias of −0.17 (95% confidence interval, −2.15 to 1.8)
To illustrate the ability of the LRMD monitor to detect apneas, the respiratory rate was recorded with the LRMD mounted to nasal prongs and by nasal capnometry while the investigator performed several breath holds (Fig. 8). Both instruments detected the apneas interspersed amongst a regular breathing pattern.
Discussion
The primary purpose of this preliminary investigation was to evaluate whether a thermodynamic temperature-based inexpensive and portable instrument (LRMD) could accurately track the respiratory rate outside the intensive care setting. First, we determined that the LRMD measurements were independent of the oxygen flow rate. Second, we determined that, whether the LRMD sensor was mounted to a face mask or nasal prongs, the tracings were synchronous and congruous with contemporaneous capnograms obtained using nasal capnometry.
The notion that a temperature-based instrument could measure indices of respiration is not a novel concept, but previous attempts to develop a viable instrument have failed for several reasons. First, rapidly responding inexpensive thermistors operating in a thermal balance circuit were not widely available in the past, thus limiting the ability of investigators to detect minute changes in temperature during the respiratory cycle. Second, the temperature profile during the respiratory cycle becomes progressively more attenuated within the microenvironment of a face mask as thermal equilibrium is reached. The blunted signal rendered a temperature-based instrument ineffective. To overcome this problem, we added a second thermistor within the thermal balance circuit to detect temperature changes throughout the respiratory cycle that were independent of the microenvironment. We then added a reference thermistor outside the face mask or nasal prongs to account for gross fluctuations in the ambient temperature. When combined with a responsive algorithm, temperature-based measurements of respiration were reliable and precise.
Currently, respiration is not widely monitored once patients leave critical care settings such as the operating room. This is a major risk for hypoxia-induced adverse outcomes. The technology available to monitor respiration suffers several limitations, including the size and cost of existing monitors. The LRMD is a lightweight instrument that may weigh as little as 10 g. The incremental disposable cost of the LRMD is approximately US$4, with an estimate for a freestanding monitor, based on the production of 1,000 units, of approximately US$250. A decrease in the cost of respiratory monitoring by several orders of magnitude, as we expect with LRMD, may introduce a paradigm shift in the ubiquity of such monitors outside the operating room.
It may seem paradoxical that this temperature-based respiratory sensor could detect “respiration” using laboratory bellows with gas flowing at room temperature. When humans breathe, the sensor detects the warm temperature in the exhaled breath as respiration. Nevertheless, we observed that, when the sensor was mounted to the face mask, the temperature within the mask equilibrated with that of the exhaled breath after several breaths, obscuring the signal detected by the sensor. To ensure that the sensor could detect respiration in the long term, we applied the Peltier-based microbalance to our sensor design. This offsets the temperature setpoint to ensure a detectable response to breathing irrespective of the ambient temperature. Hence, when air at room temperature flowed past the sensor, the Peltier effect ensured that the sensor detected respiration irrespective of the local temperature.
This study suffers from several limitations. First, these are preliminary laboratory data that serve to establish the feasibility and scope of respiratory monitoring with an instrument using a temperature-based monitoring system. Second, we studied the LRMD sensor in several investigators, not in patients. To validate this novel instrument, clinical studies are required in patients with a wide range of diseases and during a range of procedures. Currently, four clinical studies in various stages of completion are being conducted to evaluate the reliability and accuracy of the LRMD to monitor respiration in patients—one such study has been presented.Footnote 3 Third, variation in human physiology, including age, weight, and breathing patterns, must be investigated to ensure the current sensor is sufficiently robust to track respiration. Fourth, although we have preliminary evidence that the signal from the LRMD sensor is very stable during rapid movement and for prolonged periods (www.Linshomforlife), additional testing is needed to verify these findings in patients. Many of these limitations will be addressed in the clinical studies that are underway.
In summary, this concept paper presents preliminary data to show that this novel temperature-based respiratory instrument, the Linshom Respiratory Monitoring Device, tracks respiration noninvasively. Our results show that, when the LRMD sensor is mounted to a face mask or nasal prongs, the instrument can accurately measure the respiratory rate over a wide range. These findings warrant corroboration in clinical trials and in patients under diverse conditions.
Notes
US Patents #8,579,829 and non-provisional approval of application #13/553,070.
Sathyamoorthy M, Lerman J, Feldman D, et al. Linshom. a new respiratory monitor. American Society of Anesthesiologists’ Meeting, San Francisco, CA. 2013: A5032
Preiss D, Drew B, Gosnell J, Kodali BS, Philip JH. A thermodynamic breathing sensor—a new non-invasive monitor of respiration. American Society of Anesthesiologists, San Diego, October 2015, BOC09.
References
Manifold CA, Davids N, Villers LC, Wampler DA. Capnography for the nonintubated patient in the emergency setting. J Emerg Med 2013; 45: 626-32.
Brouillette RT, Morrow AS, Weese-Mayer DE, Hunt CE. Comparison of respiratory inductive plethysmography and thoracic impedance for apnea monitoring. J Pediatr 1987; 111: 377-83.
Ramsay MA, Usman M, Lagow E, Mendoza M, Untalan E, De Vol E. The accuracy, precision and reliability of measuring ventilatory rate and detecting ventilatory pause by rainbow acoustic monitoring and capnometry. Anesth Analg 2013; 117: 69-75.
Keidan I, Gravenstein D, Berkenstadt H, Ziv A, Shavit I, Sidi A. Supplemental oxygen compromises the use of pulse oximetry for detection of apnea and hypoventilation during sedation in simulated pediatric patients. Pediatrics 2008; 122: 293-8.
Williamson JA, Webb RK, Cockings J, Morgan C. The Australian Incident Monitoring Study. The capnograph: applications and limitations-an analysis of 2000 incident reports. Anaesth Intensive Care 1993; 21: 551-7.
Nassi N, Piumelli R, Lombardi E, Landini L, Donzelli G, de Martino M. Comparison between pulse oximetry and transthoracic impedance alarm traces during home monitoring. Arch Dis Child 2008; 93: 126-32.
Kasuya Y, Akça O, Sessler DI, Ozaki M, Komatsu R. Accuracy of postoperative end-tidal Pco2 measurements with mainstream and sidestream capnography in non-obese patients and in obese patients with and without obstructive sleep apnea. Anesthesiology 2009; 111: 609-15.
Farré R, Montserrat JM, Rotger M, Ballester E, Navalas D. Accuracy of thermistors and thermocouples as flow-measuring devices for detecting hypopneas. Eur Respir J 1998; 11: 179-82.
Funding
No external funding was received for conducting this investigation or for analyzing these data.
Conflicts of interest
Five authors (J. Lerman, D. Feldman, R. Feldman, J. Moser, and U. Feldman) jointly hold two patents in the U.S.A. that were issued for this device: #8,579,829 and non-provisional patent approved #13/553,070. The remaining authors do not hold any interests in this instrument that might be perceived as conflicts of interest.
Author contributions
Jerrold Lerman, Doron Feldman, Ronen Feldman, Kenneth Deitch, and Uri Feldman participated in the study design. Jerrold Lerman, Doron Feldman, Ronen Feldman, John Moser, Leeshi Feldman, Madhankumar Sathyamoorthy, and Uri Feldman participated in the study execution. Jerrold Lerman, Doron Feldman, Ronen Feldman, Leeshi Feldman, Madhankumar Sathyamoorthy, and Uri Feldman participated in data acquisition. Jerrold Lerman, Ronen Feldman, John Moser, and Uri Feldman participated in the data analysis. Jerrold Lerman, Doron Feldman, Ronen Feldman, John Moser, Kenneth Deitch, and Uri Feldman participated in study interpretation. Jerrold Lerman and Ronen Feldman participated in figure production. Jerrold Lerman, Doron Feldman, Ronen Feldman, and John Moser participated in manuscript writing. Jerrold Lerman, Doron Feldman, Ronen Feldman, John Moser, Leeshi Feldman, Madhankumar Sathyamoorthy, Kenneth Deitch, and Uri Feldman participated in manuscript editing. John Moser participated in the hardware design. Uri Feldman participated in development of the algorithm. Jerrold Lerman takes full responsibility for the integrity of the data/analyses presented.
Editorial responsibility
This submission was handled by Dr. Gregory L. Bryson, Deputy Editor-in-Chief, Canadian Journal of Anesthesia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lerman, J., Feldman, D., Feldman, R. et al. Linshom respiratory monitoring device: a novel temperature-based respiratory monitor. Can J Anesth/J Can Anesth 63, 1154–1160 (2016). https://doi.org/10.1007/s12630-016-0694-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-016-0694-y