Abstract
Purpose
This article presents a summary of recent advances, including tools and interventions, that are designed to improve drug safety for patients in critical care settings, particularly those undergoing anesthesia and surgery.
Principal findings
Medication error remains a leading cause of adverse events among patients undergoing anesthesia. Misidentification of ampoules, vials, and syringes is a common source of error. Systems are now being engineered to reduce the likelihood of medication misidentification through approaches such as revision of standards for labelling of drug ampoules and vials and the development of bar code systems that allow “double checking” or drug verification in the operating room. Also, efforts are being made to improve medication reconciliation, a process for accurately communicating a patient’s medication information during transitions from one healthcare setting to another. Finally, the opportunity exists for anesthesiologists to increase awareness about the rising problem of opioid addiction in patients for whom typical doses are initially prescribed for appropriate indications such as postoperative pain.
Conclusions
There is a need to improve drug delivery systems in complex critical care environments, particularly the operating room. Anesthesiologists must continue to play a leading role in promoting drug safety in these environments.
Résumé
Objectif
Cet article résume les progrès récents, y compris les outils et les interventions, conçus pour améliorer la sécurité des médicaments chez les patients dans des contextes critiques, particulièrement ceux qui subissent une anesthésie et une chirurgie.
Constatations principales
Les erreurs médicamenteuses demeurent l’une des causes principales des effets secondaires indésirables chez les patients subissant une anesthésie. La mauvaise identification des ampoules, des flacons et des seringues est une source fréquente d’erreurs. Des systèmes sont désormais mis au point afin de réduire la probabilité de mauvaise identification des médicaments via des approches telles que la révision des normes d’étiquetage des ampoules et flacons de médicaments et la mise au point de systèmes de codes à barres qui permettent la « double vérification » ou la vérification des médicaments dans la salle d’opération. En outre, des efforts sont faits pour améliorer le bilan comparatif des médicaments, un processus de communication précise des informations sur les médicaments d’un patient pendant son transfert d’un cadre de soins de santé à un autre. Enfin, il existe la possibilité, pour les anesthésiologistes, d’accroître la prise de conscience concernant le problème croissant d’accoutumance aux opiacés chez des patients à qui on prescrit initialement des doses habituelles pour des indications adaptées telles que la douleur postopératoire.
Conclusion
Il existe un besoin constant d’améliorer les systèmes de distribution des médicaments dans des environnements de soins critiques complexes, particulièrement la salle d’opération. Les anesthésiologistes doivent jouer un rôle de chef de file dans la promotion de la sécurité des médicaments dans ces environnements.
Similar content being viewed by others
Anesthesiologists generally have excellent skills in problem-solving, decision-making, and implementation. Nevertheless, when performing tasks, anesthesiologists occasionally lose their focus and make mistakes. In everyday life, such “slips” are generally inconsequential; however, in the critical care environment, they can lead to serious adverse events. The goal of the patient safety movement in anesthesiology is to engineer error-prevention strategies into care delivery systems. In addition, a variety of efforts are being made to ensure errors are immediately recognized when they do occur so as to minimize their impact.
Medication error is a leading cause of preventable adverse events in hospital inpatients. Results of both international and Canadian studies have shown high overall rates of preventable medication error.1-4 An estimated 3-6% of hospital stays are associated with adverse drug events, and 30-40% of adverse drug events result from preventable errors; such errors lead to patient suffering and an escalation of healthcare costs.1,2,5 Footnote 1 In 2007, the U.S. Institute of Medicine reviewed the causes and incidence of medication errors and concluded that the rates and effects of errors are likely markedly underestimated.2 The “victims” of medication error include not only patients, but also healthcare practitioners, medical institutions, and the public’s trust in healthcare systems.6
The focus of this current review is on strategies that are currently being engineered into care delivery systems to reduce drug-related errors in anesthetic practice. Such errors, which occur at a rate of about 1 per 130-450 patients,7-11 are associated with increases in morbidity, mortality, and the costs of hospital care.8-12 Fortunately, most anesthetic-related errors are inconsequential; however, some lead to substantial or permanent injury and even death.8,13 In this review, we focus on systems designed to reduce medication errors in the operating room and highlight three Canadian initiatives: 1) systematic efforts to improve the labelling of drug ampoules and vials, 2) introduction of bar-coding in medication systems, and 3) novel tools for “reconciliation” or for accurate documentation and adjustment of patients’ medication taken before and during their hospital stay. This article concludes with a description of emerging issues for drug safety in anesthesia. Readers interested in more comprehensive reviews of medication errors in anesthetic practice are referred to other articles12,14 and recommendations.14-18
Medication safety in anesthesia
The specialty of anesthesiology has been referred to as the “ODAM” profession because anesthesiologists are required to order, dispense, administer, and monitor high-risk drugs while performing a variety of additional tasks in a complex work environment. Safety experts refer to nearly all anesthesia-related medications as “high-alert” drugs because their misuse has the potential to cause serious harm. Moreover, in the operating room, strategies that are used to reduce drug errors in other critical care environments (e.g., automated warnings from computerized physician order-entry systems or double-checks with a second person) may be either impractical or unavailable. Consequently, it is not surprising that medication errors occur but rather, they occur so infrequently.
Based on a limited number of prospective studies, the estimated incidence of medication error in anesthetic practice ranges from 0.33-0.73%,7,9 and the rate has not changed substantially in over 15 years.11 Despite a growing awareness of the importance of drug safety, medication error is a major cause of medical malpractice for anesthesiologists in Canada.19
The cause of a medication error during anesthesia is often multifactorial with clear patterns emerging from analyses of large error-reporting databases. Syringe swaps, more formally known as syringe substitution errors, represent the most frequent type of error. This problem is often due to administration of an incorrect drug from a correctly labelled syringe.11,13,20 Switching of look-alike drug ampoules and vials is also common. The drugs most frequently involved include neuromuscular blocking agents, inotropes, and opioids.11,13,20 Interestingly, many errors occur during the maintenance phases of anesthesia, possibly when the anesthesiologist’s vigilance is low. The level of experience of the anesthesia care providers is another major contributing factor, given that the error rate is almost twice as high among trainees as among experienced anesthesiologists.7,11
Evidence-based recommendations intended to minimize errors have been developed on the basis of a systematic review.12 The most important recommendation is the implementation of system-based interventions to reduce drug errors during anesthesia. Here, we discuss system-based interventions to improve the labels on drug ampoules and to introduce bar-coding systems.
Labels on drug ampoules and vials
Misidentification is a common source of error, which suggests that improvements in the labelling of drug ampoules and vials are essential to reduce error rates.10,21 The Canadian Standards Association (CSA) developed the first Canadian standard for labelling of drug ampoules, vials, and prefilled syringes, building on previous work by the Canadian Society of Hospital Pharmacists and input from Health Canada.Footnote 2 , Footnote 3 The CSA standard is designed to complement Health Canada regulations and guidance for labelling of pharmaceuticals. The Institute for Safe Medication Practices (ISMP) Canada (www.ismp-canada.org) is currently engaged in a collaborative initiative with Health Canada to develop labelling and packaging guidelines specifically designed to optimize patient safety. The project is seeking input from many stakeholders, including the Canadian Anesthesiologists’ Society, and it is aligned with international efforts to improve labels and packaging. The International Medication Safety NetworkFootnote 4 is also working to ensure that national and international standards for drug labels will be aligned, including aspects such as minimum font size, colour, and the specific information to be presented (e.g., expression of drug strength and total drug content in a vial).
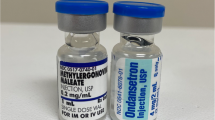
Over the past five years, labelling and packaging were major contributing factors in nearly 500 reports of errors to ISMP Canada. On the basis of these reports, improvements have been developed for specific groups of high-risk compounds. For example, labelling of neuromuscular blocking drugs has been changed in response to reported medication incidents.22,23 Since 2008, all manufacturers have voluntarily included warnings on the caps and ferrules of vials of neuromuscular blocking agents sold in Canada.24 No reports of mix-ups between neuromuscular blocking agents and other medications have been received by ISMP Canada since the changes were implemented (although it is not possible to infer or project the probability of specific incidents on the basis of a voluntary error reporting program). The United States Pharmacopeia is now developing a standard that will allow only cautionary warnings, such as “Paralyzing Agent” on the cap or ferrule of a drug vial (Fig. 1).25 Designing labels with safety in mind is one step forward toward building a safer system. Additional system safeguards, such as automated identification (bar-coding) to assist with drug identification, described below, will be key steps in the future.
Substitution errors involving syringes, more commonly referred to as “syringe swaps”, have led to recommendations for the use of colour-coded self-adhesive labels to ensure correct labelling and identification of syringes. The CSA has adopted the recommendations of the International Organization for Standards (ISO) for user-applied labels for syringes that contain drugs to be administered during anesthesia,Footnote 5 and this serves as the Canadian standard.Footnote 6 It has been suggested that labels should be applied to syringes before the drugs are drawn up. Others have argued that the label should be applied only after the drug has been drawn up. The important point is that there should be no interruption in workflow from the time the drug is drawn up and labelled to the time the syringe leaves the anesthesiologist’s hand. Furthermore, the legibility of the drug concentration written on the label should be optimized, and the workspace, including counters and drawers housing the syringes, should be well organized and standardized. Finally, several manufactures now produce prefilled syringes for use during general anesthesia. Use of these syringes would prevent several of the common errors that result from incorrect filling or labelling of syringes. Some hospitals are developing their own system involving the use of prefilled syringes to reduce the risk of error but also to address national and international shortages of anesthetic-related drugs.
Bar-coding (automated identification)
According to one study, double-checking the labels on drug ampoules, vials, and syringes could prevent 58% of all drug-related errors.26 Unfortunately, double-checking all anesthesia-related drugs in the busy complex work environment of the operating room is generally impractical and distracting and could lead to error. Nevertheless, bar-coding, which has been shown to reduce preventable medication errors by 50% in other acute and critical care environments, may prove helpful in the operating room.26-28 Footnote 7
The first step in creating a safe bar-coding system for the operating room is the development of national standards for the drug product bar codes themselves. Standards are also required for the devices and software that will be used to scan, interpret, and document the codes. Unfortunately, Canada currently has no federal regulations or guidance for bar-coding pharmaceutical products. Consequently, a collaboration of six healthcare sectors has taken the initiative to develop a voluntary national standard for the use of bar codes on commercial pharmaceutical products. The Canadian Pharmaceutical Bar Coding Project began in 2008, with endorsement by the Canadian Anesthesiologists’ Society in July 2011.29 In February 2012, the (Version II) Joint Technical Statement on Pharmaceutical Automated Identification and Product Database Requirements was published. This document provides nationally accepted recommendations for the use of automated identification standards.30 In addition to presenting recommendations for a national standard, the Canadian Pharmaceutical Bar Coding Project has defined guidelines for the placement of bar codes on pharmaceutical labels for primary packaging31 as well as guidelines for the minimum software features that will be required and a checklist for institutions that are considering adopting these new information systems.32
In the future, anesthesiology practice should benefit from efforts to develop a common approach to the automated identification of medications. The goal of a national automated identification standard would be to ensure the safe flow of drugs along the entire medication chain, from Health Canada’s approval of commercial pharmaceutical products to their use by anesthesiologists or by nurses who care for patients following anesthesia (Fig. 2).
The next major hurdle will be the development of guidelines for automated identification of drugs within specific point-of-care settings. Customization of specific anesthesiology processes will be required to accommodate specialized dose formats established by the manufacturers or by the institutions themselves (e.g., preferred concentrations of a particular drug). Such pharmacy-prepared dilutions would still be subject to the requirements outlined in the CSA standard for user-applied labels.6
Any automated systems and devices must be tested for ease of use and must not distract from other tasks. Such systems could add medication management information to patient electronic records. The process of automated identification in the operating room is gradually moving forward, but a great deal of developmental work remains to be done (Fig. 3).33,34
One example of the incorporation of automated identification into anesthesia care is the SAFERsleep®, a multimodal system developed by Merry et al., which is designed specifically to reduce medication errors associated with anesthesia care.33 Along with the automated identification system for use in the operating room, the system includes customized drug trays, prefilled syringes containing the most commonly used drugs, colour-coded drug labels and a bar code reader coupled with a system for auditory and visual verification. Drugs can be checked before administration, and the correct drug name and dose can then be documented in the electronic patient record. A prospective randomized open-label clinical trial was undertaken in a tertiary care hospital in New Zealand to evaluate the impact of the SAFERsleep system on errors in the recording and administration of drugs and on anesthesiologists’ vigilance and workload.
The SAFERsleep system was compared with conventional drug management methods. The primary outcome measure was a composite score of errors in the recording and administration of intravenously administered drugs. Drug errors were detected both by direct observation and by a detailed check of the drug containers used during each procedure. In addition, the number of lapses in response to a vigilance latency test was measured for each anesthesiologist. Secondary outcomes included patient outcomes, anesthesiologists’ workload, and legibility of anesthesia patient records. The study also included an assessment of compliance with the procedural rules of the new system and a report on the results of a questionnaire completed by the participants.
The mean rate of drug error was 11.6 per 100 administrations with the conventional practice and 9.1 per 100 administrations with the SAFERsleep system, a difference of 2.5 per 100 drug administrations or 21%. In both situations, most of the errors were recording errors. Electronic records generated by the SAFERsleep system were more legible, and anesthesiologists preferred the new system for long, complex, and emergency cases. There were no differences in patient outcomes or anesthesiologists’ workload. Interestingly, anesthesiologists spent less time observing the monitor when the SAFERsleep system was in use, and there were more lapses in a vigilance task.
In a similar study at the University Health Network hospitals in Toronto, Canada, the authors assessed a new process for dispensing, labelling, and verifying drugs for use in the operating room.34 A device called the DuoCheck™ was designed to scan bar codes and provide audible and visual feedback to confirm the drug name. The device, which also generates labels and allergy alerts, was coupled with a newly established medication preparation and administration workflow process. No errors were documented during the trial period, and anesthesiologists reported high satisfaction and minimal interruptions of workflow associated with the DuoCheck device.34
The development of devices like these, intended to “double-check” medications in the operating room, is still in the pioneering stages. Nevertheless, it is expected that such tools, coupled with electronic patient records, will be adopted more widely in the not-so-distant future.
Medication reconciliation
Medication reconciliation programs aim to ensure accurate communication of patients’ medication information during transitions from one healthcare setting to another. The goal of such programs is to develop systematic processes that will prevent omissions and duplications of medications as well as inappropriate therapy. For example, in the context of anesthesia care, it is essential to document whether patients are receiving anticoagulant or antihypertensive therapies in the preoperative period and to plan if and when these drugs should be discontinued before surgery and then resumed afterward. Interventions may be required to improve the documentation and reconciliation of medications, particularly during the perioperative period. Such interventions could prevent adverse drug events that commonly occur in elderly patients following discharge from hospital, including failure to start taking new medications and incorrect resumption of medications discontinued in hospital.35 For example, patients receiving long-term warfarin therapy are at risk for unintended medication discontinuation after elective ambulatory procedures and even after overnight hospital stays.36
In one study, medication reconciliation was reported to reduce the rate of medication errors from 213 per 100 admissions to 63 per 100 admissions.37 Ideally, patients and their families are closely involved in medication reconciliation and should receive complete and accurate information about their medications during the perioperative period. A variety of reconciliation programs for reviewing medications before surgery have been investigated. For example, combining pharmacist assessments of medication in a preoperative preadmission clinic and use of a postoperative medication order form reduced the number of medication discrepancies.38 The most common error was failure to order home medications in the postoperative period. In another project, linking Ontario MedsCheck Program community pharmacy-based medication records with pre-op assessments before elective orthopedic surgery39 resulted in improved preoperative medication assessments.40 Furthermore, careful medication reconciliation at discharge has been shown to reduce preventable adverse drug events, which not only enhances patient safety but could also reduce healthcare costs.41
In addition to the tools and processes for institution-based medication reconciliation, patient-centred interventions are now available. For example, an iPhone application, MyMedRec, helps patients track their medication use.42 The ability to integrate provincial drug programs (which acts as a central repository for patient prescription information) with admission records could provide another solution.
To date, the medication reconciliation processes have been implemented only on a limited scale.43 This slow uptake of programs may result from the complexity of the intervention and the need to build expertise and resources for proper implementation. Implementation is also highly dependent on communication between various groups or teams of caregivers. Advances in technology, such as the development of robust computerized electronic medical records, will likely improve the availability of medication information at transitions of care, thus facilitating medication reconciliation.
Reporting and learning from medication incidents
The Canadian Medication Incident Reporting and Prevention System (CMIRPS) is a collaborative program that receives reports from healthcare facilities, individual practitioners, and, more recently, consumers.44 Key stakeholders, including the Canadian Anesthesiologists’ Society, have visibly supported the CMIRPS program through the dissemination of safety bulletins detailing recommendations for risk mitigation activities and best practices for safe use of medications.45 Various provincial programs have also supported CMIRPS. For example, as of October 2011, all Ontario hospitals are required to report critical incidents involving medications and intravenous fluids to the Canadian Institute for Health Information, a key partner in the CMIRPS program.46 Anesthesiologists can report adverse drug events or potential events through an anonymous Web site portal https://www.ismp-canada.org/err_report.htm.
Safer use of opioids in pain management
Anesthesiologists could play an important role in the safer use of opioids in the treatment of pain. Canada is the second largest consumer of prescription opioids on a per capita basis after the United States.47 According to the U.S. Centers for Disease Control and Prevention, excessive prescribing of opioid analgesics is fuelling an epidemic of narcotic addiction and overdose deaths.48 The introduction of long-acting opioid products has been associated with increases in the daily dose of opioids, and high daily doses are strongly associated with opioid-related mortality.49
Opioid addiction can and does occur with typical doses that are prescribed for approved indications, and this problem is not rare. Dependence and addiction are relatively common consequences of long-term opioid therapy, occurring in up to one-third of patients in some studies.Footnote 8 A more balanced approach to the treatment of non-malignant pain is needed, one that includes careful assessment of comorbidities and consideration of options for non-narcotic and/or non-pharmacologic therapy. In addition, new ways of thinking about the problem are needed. For example, opioid addiction resulting from escalated use of prescribed drugs could be viewed as an adverse drug reaction or a preventable event. With their established leadership role in pain management, anesthesiologists are well positioned to promote awareness about the rising problem of narcotic addiction, to help in preventing medication-related adverse events, and to lead improvements in the use of opioids.
Improved identification and management of drug interactions
A developing area of interest for anesthesiologists is drug-drug interactions. One example reported to CMIRPS was a preventable interaction between fentanyl patch (applied by patch) and antiretroviral medications that resulted in death.50 Certain antihypertensive drugs that are commonly used for patients with congestive heart failure, diabetic nephropathy, and hypertension, including angiotensin converting enzyme inhibitors and angiotensin receptor blockers, increase the risk of severe and refractory hypotension under general anesthesia.51,52 Stopping these drugs the day before surgery may reduce the risk of serious intraoperative hypotension.53 Similarly, if various anticoagulants that are used in the treatment of stroke, atrial fibrillation, and venous thrombosis are not identified in the preoperative period, they may pose a serious risk for procedures such as regional anesthesia. Overall, with the steadily increasing number of drug products (now more than 20,000) in Canada, the opportunities for harmful drug interactions are likely to increase.54
Best practice consensus guidelines for disease management are often widely available; but only limited guidance is available regarding the management of patients with multiple comorbidities requiring complex medication therapy. As a result, there is an overreliance on human memory to identify and resolve possible drug interactions. To address this problem, point-of-care information systems are needed to allow detection of potentially harmful drug interactions or contraindications before the drugs are administered. For example, pharmacological databases that provide point-of-care warnings of potential drug interactions are now readily available for download to handheld devices (e.g., www.epocrates.com). In addition, some hospital pharmacies offer computerized drug interaction programs that generate certain alerts. Then again, such systems are not entirely reliable; in one evaluation, they failed to detect up to a third of drug-drug interactions but frequently alerted users to trivial issues.55
Conclusion
In summary, as both the breadth of our pharmacological armamentarium and the complexity of anesthesia care environments increase, re-engineering of drug delivery systems is required to prevent harm. Organizations, such as the Canadian Anesthesiologists’ Society, the Anesthesia Patient Safety Foundation, and ISMP Canada among many others, are collaborating with Health Canada and international organizations to implement strategies that will safeguard patients from medication-related harm.
Key points
-
Medication error is a leading cause of preventable adverse events in hospitalized patients.
-
Medication errors in anesthetic practice occur at an approximate rate of 1 per 130-450 patients.
-
Adverse drug events are a leading cause of medico-legal concern for Canadian anesthesiologists.
-
Misidentification of ampoules and vials and inadvertent “syringe swaps” are common causes of error.
-
To prevent misidentification, improvements in the standards for labels on drug containers are required.
-
The use of self-adhesive colour-coded labels for syringes is recommended, and readers are referred to the latest CSA/ISO standard for user-applied labelling of medication containers and syringes within anesthesiology practice.
-
Bar code software systems that “double-check” drug vials and syringes are being developed for use in the operating room to support drug verification during procedures and provide improved documentation of health records.
-
Medication reconciliation is a process to improve communication of medication information at transitions of care.
-
Anesthesiologists, in their leadership role in pain management, are well positioned to influence improved use of opioid medications in non-malignant pain.
Notes
National Health Service (U.K.): Institute for innovation and improvement. Reducing avoidable deaths: chief executives. June 2007.
CAN/CSA-Z264.2-99 (R2009) Labelling of drug ampoules, vials, and prefilled syringes. Mississauga, (ON): CSA Group; 1999 [reaffirmed 2009].
Drug packaging and labelling guidelines for manufacturers. (ON) Canadian Society for Hospital Pharmacists: 2001.
“International Medication Safety Network [homepage]; Horsham (PA)” Available from http://www.intmedsafe.net/Contents/Home.aspx.
ISO 26825:2008, Anaesthetic and respiratory equipment – user-applied labels for syringes containing drugs used during anesthesia – colours, design and performance. Geneva (Switzerland): International Organization for Standardization; 2008.
CAN/CSA-ISO 26825-10, Anaesthetic and respiratory equipment – user-applied labels for syringes containing drugs used during anesthesia – colours, design and performance. Mississauga (ON): CSA Group; 2010.
Wideman MV, Whittler ME, Anderson TM. Barcode medication administration: lessons learned from an intensive care unit implementation. Advances in patient safety: from research to implementation (Volume 3: Implementation issues). Rockville (MD): Agency for Healthcare Research and Quality (US); 2005 Feb.
Juurlink DN, Dhalla I. Dependence and addiction during chronic opioid therapy. J Med Toxicol. E-pub ahead of Print. October 2012.
References
Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ 2004; 170: 1678-86.
Committee on Identifying and Preventing Medication Errors; Aspden P, Wolcott J, Bootman JL, Cronenwett LR. Board on Health Care Services. Preventing Medication Errors: Quality Chasm Series. The National Academies Press 2007. Available from URL: http://www.nap.edu/catalog.php?record_id=11623 (accessed November 2012).
Pronovost PJ, Miller MR, Wachter RM. Tracking progress in patient safety: an elusive target. JAMA 2006; 296: 696-9.
Auerbach AD, Landefeld CS, Shojania KG. The tension between needing to improve care and knowing how to do it. N Engl J Med 2007; 357: 608-13.
Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA 1995; 274: 29-34.
Denham CR. TRUST: The 5 rights of the second victim. J Patient Saf 2007; 3: 107-19.
Cooper L, DiGiovanni N, Schultz L, Taylor AM, Nossaman B. Influences observed on incidence and reporting of medication errors in anesthesia. Can J Anesth 2012; 59: 562-70.
Webster CS, Merry AF, Larsson L, McGrath KA, Weller J. The frequency and nature of drug administration error during anaesthesia. Anaesth Intensive Care 2001; 29: 494-500.
Zhang Y, Dong YJ, Webster CS, et al. The frequency and nature of drug administration error during anaesthesia in a Chinese hospital. Acta Anaesthesiol Scand 2012; DOI: 10.1111/j.1399-6576.2012.02762.x.
Fasting S, Gisvold SE. Adverse drug errors in anesthesia, and the impact of coloured syringe labels. Can J Anesth 2000; 47: 1060-7.
Sakaguchi Y, Tokuda K, Yamaguchi K, Irita K. Incidence of anesthesia-related medication errors over a 15-year period in a university hospital. Fukuoka Igaku Zasshi 2008; 99: 58-66.
Jensen LS, Merry AF, Webster CS, Weller J, Larsson L. Evidence-based strategies for preventing drug administration errors during anaesthesia. Anaesthesia 2004; 59: 493-504.
Orser BA, Chen RJ, Yee DA. Medication errors in anesthetic practice: a survey of 687 practitioners. Can J Anaesth 2001; 48: 139-46.
Wheeler SJ, Wheeler DW. Medication errors in anaesthesia and critical care. Anaesthesia 2005; 60: 257-73.
Currie M, Mackay P, Morgan C, et al. The Australian Incident Monitoring Study. The “wrong drug” problem in anaesthesia: an analysis of 2000 incident reports. Anaesth Intensive Care 1993; 21: 596-601.
Orser BA, Oxorn DC. An anaesthetic drug error: minimizing the risk. Can J Anaesth 1994; 41: 120-4.
Merry AF, Peck DJ. Anaesthetists, errors in drug administration and the law. N Z Med J 1995; 108: 185-7.
Orser BA. Medication safety in anesthetic practice: first do no harm. Can J Anesth 2000; 47: 1051-4.
Orser BA, Byrick R. Anesthesia-related medication error: time to take action. Can J Anesth 2004; 51: 756-60.
Abeysekera A, Bergman IJ, Kluger MT, Short TG. Drug error in anaesthetic practice: a review of 896 reports from the Australian Incident Monitoring Study database. Anaesthesia 2005; 60: 220-7.
Short TG, O’Regan A, Lew J, Oh TE. Critical incident reporting in an anaesthetic department quality assurance programme. Anaesthesia 1993; 48: 3-7.
The Institute for Safe Medication Practices Canada. ISMP Canada Safety Bulletin, 2006; 6(2): 2, April 25. Neuromuscular Blocking Agent Labelling and Packaging Initiative. Available from URL: http://www.ismp-canada.org/download/safetyBulletins/ISMPCSB2006-02PotassiumPhosphates.pdf (accessed November 2012).
The Institute for Safe Medication Practices Canada. ISMP Canada Safety Bulletin, 2004; 4: 1-2. “Paralyzing” Mix-Ups in the Operating Room: Opportunity to Improve Safety with Neuromuscular Blockers. Available from http://www.ismp-canada.org/download/safetyBulletins/ISMPCSB2004-07.pdf (accessed November 2012).
The Institute for Safe Medication Practices Canada. ISMP Canada Safety Bulletin 2010; 10(3):4, May 25. Neuromuscular Blocking Agents: Another Manufacturer Changes Labelling and Packaging to Increase Safety. Available from URL: http://www.ismp-canada.org/download/safetyBulletins/ISMPCSB2010-03-KnowledgeTranslation.pdf (accessed November 2012).
The Institute for Safe Medication Practices Canada. ISMP Canada Safety Bulletin 2011; 11(7):2, November 15. New USP Requirement for Drug Vial Caps and Ferrules. Available from URL: http://www.ismp-canada.org/download/safetyBulletins/ISMPCSB2011-7-Iodine_Overdose_with_Lugols_Solution.pdf (accessed November 2012).
Paoletti RD, Suess TM, Lesko MG, et al. Using bar-code technology and medication observation methodology for safer medication administration. Am J Health Syst Pharm 2007; 64: 536-43.
Poon EG, Keohane CA, Yoon CS, et al. Effect of bar-code technology on the safety of medication administration. N Engl J Med 2010; 362: 1698-707.
Morriss FH Jr, Abramowitz PW, Nelson SP, et al. Effectiveness of a barcode medication administration system in reducing preventable adverse drug events in a neonatal intensive care unit: a prospective cohort study. J Pediatr 2009; 154: 363-8.
Institute for Safe Medication Practices Canada. Canadian Pharmaceutical Bar Coding Project: Designing a National Strategy for Healthcare. Toronto (ON), ©2000-2012. Available from URL: http://www.ismp-canada.org/barcoding/index.htm (accessed November 2012).
Institute for Safe Medication Practices Canada. Canadian Pharmaceutical Bar Coding Project. Joint Technical Statement (version II) on Pharmaceutical Automated Identification and Product Database Requirements. Toronto (ON): 2012. Available from URL: http://www.ismp-canada.org/barcoding/download/JTSv2/JTSv2.pdf (accessed November 2012).
Institute for Safe Medication Practices Canada. Supplement A. Canadian Pharmaceutical Bar Coding Project: Guidance for Placement of Bar Codes on Pharmaceutical Labels for Primary Packaging. Toronto (ON): 2012. Available from URL: http://www.ismp-canada.org/barcoding/download/JTSv2/SupplA-LabellingGuidelines.pdf (accessed November 2012).
Institute for Safe Medication Practices Canada. Supplement B. Canadian Pharmaceutical Bar Coding Project: Minimum Software Safety Functionality Checklist. From the Joint Technical Statement on Pharmaceutical Automated Identification and Product Database Requirements (Version II). Toronto (ON): 2012. Available from URL: http://www.ismp-canada.org/barcoding/download/JTSv2/SupplB-MinFunctionality.pdf (accessed November 2012).
Merry AF, Webster CS, Hannam J, et al. Multimodal system designed to reduce errors in recording and administration of drugs in anaesthesia: prospective randomized clinical evaluation. BMJ 2011; DOI: 10.1136/bmj.d5543.
Fung E. Point-of-care medication bar-coding system in the OR. Pharmacy Practice 2009; (April): 1-6.
Gray SL, Mahoney JE, Blough DK. Adverse drug events in elderly patients receiving home health services following discharge. Ann Pharmacother 1999; 33: 1147-53.
Bell CM, Bajcar J, Bierman AS, Li P, Mamdani MM, Urbach DR. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Intern Med 2006; 166: 2525-31.
Rozich JD, Resar RK. Medication safety: one organization’s approach to the challenge. J Clin Outcomes Manage 2001; 8: 27-34.
Kwan Y, Fernandes OA, Nagge JJ, et al. Pharmacist medication assessments in a surgical preadmission clinic. Arch Intern Med 2007; 167: 1034-40.
Leung V, Mach K, Charlesworth E, Hicks S, Kizemchuk K, Stumpo C. Perioperative Medication Management (POMM) pilot: integrating a community-based medication history (MedsCheck) into medication reconciliation for elective orthopaedic surgery inpatients. Canadian Pharmacists Journal 2010; 143: 82-7.
Fernandes OA. Medication reconciliation. Pharmacy Practice 2009; (October): 24-32, 52-5.
Schnipper J, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med 2006; 166: 565-71.
ISMP Canada. Knowledge is the Best Medicine: iPhone app. Ottawa (ON): Rx&D; 2012. Available from URL: http://www.knowledgeisthebestmedicine.org/index.php/en/iphone_app (accessed November 2012).
Accreditation Canada. How safe are Canadian health organizations? 2011 Report on Required Organizational Practices. Ottawa (ON): 2011. Available from URL: http://www.accreditation.ca/uploadedFiles/News_and_Publications/Publications/Report_on_ROPs/2011-Report-on-ROPs.pdf (accessed November 2012).
Canadian Medication Incident Reporting and Prevention System. Home Page; CMIRPS – SCDPIM; 2011. Available from URL: http://www.cmirps-scdpim.ca/?p=14 (accessed November 2012).
Canadian Anesthesiologists’ Society. Safety Alerts, 2006-2012. Available from URL: http://www.cas.ca/English/Safety-Alerts (accessed November 2012).
Ontario Ministry of Health and Long-Term Care. Excellent Care for All, 2011. Available from URL: http://www.health.gov.on.ca/en/ms/ecfa/public/default.aspx (accessed November 2012).
Canadian Centre on Substance Abuse. International Narcotics Control Board 2010. Addressing Prescription Drug Misuse in Canada. National Dialogue Summary Report. Available from URL: http://www.ccsa.ca/2012%20CCSA%20Documents/2012-Prescription-Drug-Misuse-in-Canada%E2%80%93Summary-Report-en.pdf (accessed November 2012).
Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses — a U.S. epidemic. Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report 2012; 61: 10-3.
Gomes T, Mamdami MM, Dhalla I, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med 2011; 171: 686-91.
The Institute for Safe Medication Practices Canada. (ISMP Canada Safety Bulletin). 2008; 8(3): 1-2. Drug Interaction Incident with HIV Post-Exposure Prophylaxis. Available from URL: http://www.ismp-canada.org/download/safetyBulletins/ISMPCSB2008-03HIVPEP.pdf (accessed November 2012).
Lange M, Van Aken H, Westphal M, Morelli A. Role of vasopressinergic V1 receptor agonists in the treatment of perioperative catecholamine-refractory arterial hypotension. Best Pract Res Clin Anaesthesiol 2008; 22: 369-81.
Coriat P, Richer C, Douraki T, et al. Influence of chronic angiotensin-converting enzyme inhibition on anesthetic induction. Anesthesiology 1994; 81: 299-307.
Mekontso-Dessap A, Houel R, Soustelle C, Kirsch M, Thebert D, Loisance DY. Risk factors for post-cardiopulmonary bypass vasoplegia in patients with preserved left ventricular function. Ann Thorac Surg 2001; 71: 1428-32.
Health Canada. Drugs and Health Products. Nov 7, 2012; Health Canada. October 2012. Available from URL: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/databasdon/dpd_bdpp_data_extract-eng.php and http://www.hc-sc.gc.ca/dhp-mps/prodnatur/applications/licen-prod/lnhpd-bdpsnh_data_extract-eng.php (accessed November 2012).
Hazlet TK, Lee TA, Hansten PD, Horn JR. Performance of community pharmacy drug interaction software. J Am Pharm Assoc 2001; 41: 200-4.
Acknowledgements
Dr. Orser is a Canada Research Chair in Anesthesia. Her research is supported by the Canadian Institutes of Health Research.
Competing interests
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orser, B.A., Hyland, S., U, D. et al. Review article: Improving drug safety for patients undergoing anesthesia and surgery. Can J Anesth/J Can Anesth 60, 127–135 (2013). https://doi.org/10.1007/s12630-012-9853-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-012-9853-y