Abstract
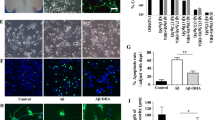
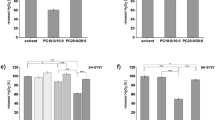
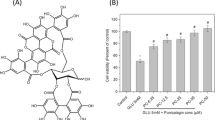
Increased levels of C22:0, C24:0 and C26:0 were found in cortical lesions of patients with Alzheimer’s disease (AD). So, it was of interest to precise the cytotoxic effects of these fatty acids, and to determine whether docosahexaenoic acid (DHA), described to prevent AD, can attenuate their eventual side effects. Human neuronal SK-N-BE cells were cultured in the absence or presence of C22:0, C24:0 or C26:0 (0.1–20 μM) without or with DHA (50–150 μM). C22:0, C24:0 and C26:0 induce an inhibition of cell growth, a loss of Δψm, an overproduction of reactive oxygen species (ROS), a decrease of reduced glutathione, and a lipid peroxidation. DHA attenuates C22:0, C24:0 and C26:0 induced-mitochondrial dysfunctions and/or cell growth inhibition measured with MTT whatever the concentrations considered, whereas it can either decrease or amplify (especially at 150 μM) ROS overproduction. C22:0, C24:0 and C26:0 have neurotoxic activities, and depending on its concentration, DHA attenuates or not fatty acid-induced side effects.
Similar content being viewed by others
References
Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9, 119–128.
Zhou ZD, Chan CH, Ma QH, Xu XH, Xiao ZC, Tan EK. The roles of amyloid precursor protein (APP) in neurogenesis: Implications to pathogenesis and therapy of Alzheimer disease. Cell Adh Migr. 2011;5, 280–292.
Kurz A, Perneczky R. Amyloid clearance as a treatment target against Alzheimer’s disease. J Alzheimers Dis. 2011;24 Suppl 2, 61–73.
Cavallucci V, D’Amelio M, Cecconi F. Aβ toxicity in Alzheimer’s disease. Mol Neurobiol. 2012;45, 366–378.
Iqbal K, Liu F, Gong CX, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 2010;7, 656–664.
Grimm MO, Rothhaar TL, Hartmann T. The role of APP proteolytic processing in lipid metabolism. Exp Brain Res. 2012;217, 365–375.
Björkhem I, Cedazo-Minguez A, Leoni V, Meaney S. Oxysterols and neurodegenerative diseases. Mol. Aspects Med. 2009;30, 171–179.
Iuliano L. Pathways of cholesterol oxidation via non-enzymatic mechanisms. Chem Phys Lipids. 2011;164, 457–468.
Vaya J, Schipper HM. Oxysterols, cholesterol homeostasis, and Alzheimer disease. J Neurochem. 2007;102, 1727–1237.
Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9, 106–118.
Titorenko VI, Terlecky SR. Peroxisome metabolism and cellular aging. Traffic. 2011;12, 252–259.
Schrader M, Fahimi HD. The peroxisome: still a mysterious organelle. Histochem Cell Biol. 2008;129, 421–440.
Lizard G, Rouaud O, Demarquoy J, Cherkaoui-Malki M, Iuliano L. Potential roles of peroxisomes in Alzheimer’s disease and in dementia of the Alzheimer’s type. J Alzheimers Dis. 2012;29, 241–254.
Santos MJ, Quintanilla RA, Toro A, Grandy R, Dinamarca MC, Godoy JA, Inestrosa NC. Peroxisomal proliferation protects from beta-amyloid neurodegeneration. J Biol Chem. 2005;280, 41057–41068.
Shi R, Zhang Y, Shi Y, Shi S, Jiang L. Inhibition of peroxisomal β-oxidation by thioridazine increases the amount of VLCFAs and Aβ generation in the rat brain. Neurosci Lett. 2012;528, 6–10.
Cimini A, Moreno S, D’Amelio M, Cristiano L, D’Angelo B, Falone S, Benedetti E, Carrara P, Fanelli F, Cecconi F, Amicarelli F, Cerù MP. Early biochemical and morphological modifications in the brain of a transgenic mouse model of Alzheimer’s disease: a role for peroxisomes. J Alzheimers Dis. 2009;18, 935–952.
Fanelli F, Sepe S, D’Amelio M, Bernardi C, Cristiano L, Cimini A, Cecconi F, Ceru MP, Moreno S. Age-dependent roles of peroxisomes in the hippocampus of a transgenic mouse model of Alzheimer’s disease. Mol Neurodegener. 2013;8: 8. doi: 10.1186/1750-1326-8-8.
Kou J, Kovacs GG, Höftberger R, Kulik W, Brodde A, Forss-Petter S, Hönigschnabl S, Gleiss A, Brügger B, Wanders R, Just W, Budka H, Jungwirth S, Fischer P, Berger J. Peroxisomal alterations in Alzheimer’s disease. Acta Neuropathol. 2011;122, 271–283.
Cai Z, Zhao B, Ratka A. Oxidative stress and β-amyloid protein in Alzheimer’s disease. Neuromolecular Med. 2011;13, 223–250.
Maruszak A, Zekanowski C. Mitochondrial dysfunction and Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35, 320–330.
Baarine M, Ragot K, Athias A, Nury T, Kattan Z, Genin EC, Andreoletti P, Ménétrier F, Riedinger JM, Bardou M, Lizard G. Incidence of Abcd1 level on the induction of cell death and organelle dysfunctions triggered by very long chain fatty acids and TNF-a on oligodendrocytes and astrocytes. Neurotoxicology 2012;33, 212- 218.
Baarine M, Andréoletti P, Athias A, Nury T, Zarrouk A, Ragot K, Vejux A, Riedinger JM, Kattan Z, Bessede G, Trompier D, Savary S, Cherkaoui-Malki M, Lizard G. Evidence of oxidative stress in very long chain fatty acid-treated oligodendrocytes and potentialization of ROS production using RNA interferencedirected knockdown of ABCD1 and ACOX1 peroxisomal proteins. Neuroscience 2012;213, 1–18.
Zarrouk A, Vejux A, Nury T, El Hajj HI, Haddad M, Cherkaoui-Malki M, Riedinger JM, Hammami M, Lizard G. Induction of mitochondrial changes associated with oxidative stress on very long chain fatty acids (C22:0, C24:0, or C26:0)-treated human neuronal cells (SK-N-BE). Oxid Med Cell Longev. 2012;2012:623257. doi: 10.1155/2012/623257.
Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115, 2774–2783.
Stark DT, Bazan NG. Neuroprotectin D1 induces neuronal survival and downregulation of amyloidogenic processing in Alzheimer’s disease cellular models. Mol Neurobiol. 2011;43, 131–138.
Tanito M, Brush RS, Elliott MH, Wicker LD, Henry KR, Anderson RE. High levels of retinal membrane docosahexaenoic acid increase susceptibility to stress-induced degeneration. J Lipid Res. 2009;50, 807–819.
Muntané G, Janué A, Fernandez N, Odena MA, Oliveira E, Boluda S, Portero-Otin M, Naudí A, Boada J, Pamplona R, Ferrer I. Modification of brain lipids but not phenotype in alpha-synucleinopathy transgenic mice by long-term dietary n-3 fatty acids. Neurochem Int. 2010;56, 318–328.
Takemoto Y, Suzuki Y, Horibe R, Shimozawa N, Wanders RJ, Kondo N. Gas chromatography/mass spectrometry analysis of very long chain fatty acids, docosahexaenoic acid, phytanic acid and plasmalogen for the screening of peroxisomal disorders. Brain Dev. 2003;25, 481–487.
Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J. Immunol. 1983;130, 1910–1917.
Rothe G, Valet G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2’,7’-dichlorofluorescin. J Leukoc Biol. 1990;47, 440–448.
Hedley DW, Chow S. Evaluation of methods for measuring cellular glutathione content using flow cytometry. Cytometry 1994;15, 349–358.
Khatoon F, Moinuddin, Alam K, Ali A. Physicochemical and immunological studies on 4-hydroxynonenal modified HSA: implications of protein damage by lipid peroxidation products in the etiopathogenesis of SLE. Hum. Immunol. 2012;73, 1132–1139.
Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA. Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Chem. 2008;283, 21837- 21841.
Pikuleva IA. Cholesterol-metabolizing cytochromes P450. Drug Metab Dispos. 2006;34, 513–520.
Singh S, Kushwah AS, Singh R, Farswan M, Kaur R. Current therapeutic strategy in Alzheimer’s disease. Eur Rev Med Pharmacol Sci. 2012;16, 1651–1664.
Vejux A, Lizard G. Cytotoxic effects of oxysterols associated with human diseases: Induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol Aspects Med. 2009;30, 153–170.
Dacks PA, Shineman DW, Fillit HM. Current evidence for the clinical use of longchain polyunsaturated n-3 fatty acids to prevent age-related cognitive decline and Alzheimer’s disease. J Nutr Health Aging. 2013;17, 240–251.
Ouellet M, Emond V, Chen CT, Julien C, Bourasset F, Oddo S, LaFerla F, Bazinet RP, Calon F. Diffusion of docosahexaenoic and eicosapentaenoic acids through the blood-brain barrier: An in situ cerebral perfusion study. Neurochem Int. 2009;55, 476–482.
Eckert GP, Lipka U, Muller WE. Omega-3 fatty acids in neurodegenerative diseases: focus on mitochondria. Prostaglandins Leukot Essent Fatty Acids. 2013;88, 105–114.
Vandal M, Alata W, Tremblay C, Rioux-Perreault C, Salem Jr N, Calon F, Plourde M. Reduction in DHA transport to the brain of mice expressing human APOE4 compared to APOE2. J Neurochem. 2014;129:516–526.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zarrouk, A., Nury, T., Riedinger, J.M. et al. Dual effect of docosahexaenoic acid (attenuation or amplification) on C22:0-, C24:0-, and C26:0-Induced mitochondrial dysfunctions and oxidative stress on human neuronal SK-N-BE cells. J Nutr Health Aging 19, 198–205 (2015). https://doi.org/10.1007/s12603-014-0518-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-014-0518-0