Abstract
We tested the hypothesis that fasting affects hypoxic ventilatory responses through metabolic changes via histamine H1 receptors. Wild-type (WT) and histamine H1 receptor knockout (H1RKO) mice were studied in fed and fasted states. In the fed WT, hypoxic-gas exposure elicited an increase and a subsequent decline in ventilation (hypoxic ventilatory decline or HVD). HVD was influenced by fasting in breathing pattern with metabolic rate. Fasting elicited hypoglycemia, a drop in R, and increases in free fatty acid and ketone bodies in the serum. In H1RKO, HVD was blunted in the fed state, but it appeared in the fasted state. There was a minimal drop in R following fasting and a low triglyceride concentration. Thus, fasting affects HVD through a change in energy mobilization from glucose to lipid metabolism. Histamine H1 receptors are involved in HVD during fed and fasted states, resulting in adaptation to the environmental conditions.
Similar content being viewed by others
Introduction
Ventilation is regulated for homeostatic demands by metabolic consequences, which varies among physiological conditions [1]. An increase in metabolic rate increases ventilation, the hypercapnic ventilatory response, and the isocapnic hypoxic ventilatory response [2, 3]. A decrease in the metabolic rate induced by caloric restriction for several days decreases the hypoxic ventilatory response in normal subjects [4]. These studies have mainly focused on ventilation and chemosensitivity to progressive hypoxia and hypercapnia.
Acute ventilatory responses to hypoxia are an initial immediate increase through peripheral chemoreceptors and a subsequent decline under the conscious state in mammals [5]. The hypoxic ventilatory decline (HVD) represents inhibition of central respiratory output rather than neuronal failure due to energy limitations during limited oxygen availability (reviewed by Neubauer et al. [6]). HVD is accompanied by a drop of \( \dot{V}{\text{O}}_{ 2} \), reducing cellular activity and energy requirements, a survival mechanism for neurons and other cells. However, it is not well understood whether HVD varies among physiological conditions affecting energy metabolism. We thought that the HVD phase may vary with changes in energy metabolism induced by fasting. Recently, we found that HVD with associated hypometabolism was modified by histamine H1 receptors, presumably through the histaminergic neuron system in the hypothalamus [7].
Histaminergic neurons are localized in the posterior hypothalamus and receive various inputs from the higher brain, and project their axons to the area of the respiratory neuronal network, including the pons and the medulla oblongata [8–10]. These histological aspects support a contribution of histaminergic neurons to respiratory control, such as chemical, thermal and circadian controls [7, 11–15]. Histaminergic neurons are also involved in other autonomic functions, such as feeding behaviors [16, 17], and thermoregulation [18, 19], which could secondarily influence respiration. These are predominantly mediated by histamine H1 receptors, rather than H2 receptors and the presynaptic H3 autoreceptor.
It has also been reported that brain histamine has a role in energy metabolism, and is involved in feeding and obesity in rodents [20]. Histamine infusion into the cerebral ventricle increases plasma glucose via H1 receptors in the brain [21]. Histamine also suppresses body fat weight and lipolysis in visceral adipose tissue via H1 receptors [22, 23]. These effects of brain histamine on energy metabolism may be relevant to metabolic rate during the fasted state. Fasting changes one process in energy metabolism, that is the stimulation of fatty acid catabolism after hypoglycemia [24]. The changes in metabolism triggered by fasting may affect \( \dot{V}{\text{O}}_{ 2} \) and \( \dot{V}{\text{CO}}_{2} \), which in turn possibly affect HVD. These findings suggest that brain histamine may have a role in controlling physiological conditions during the fasted state.
In the present study, we tested the hypotheses that metabolic changes induced by fasting affect ventilatory responses to hypoxia and that histamine H1 receptors are involved in these responses when using wild-type (WT) and histamine H1 receptor knock out (H1RKO) mice.
Materials and methods
Animals
H1RKO mice were backcrossed with C57BL/6 mice (Japan SLC, Shizuoka, Japan), and H1RKO and WT mice were maintained under pathogen-free conditions as described previously [25]. Mice were housed at approximately 24°C, exposed to a daily 12:12-h light–dark cycle (lights off at 2000 hours) and were provided with food and water ad libitum before the start of a study protocol. Male mice aged 8–11 weeks were used. All experiments were performed at an environmentally controlled temperature (24–25°C) from 1100 to 1400 hours.
Protocols
Each mouse was first examined after ad libitum feeding, allowed to recover for at least 3 days, and then examined after food restriction from 2300 to 1100 hours. Each mouse was placed in a chamber to measure lung ventilation or aerobic metabolism at 1100 hours and was allowed to acclimatize to the chamber for at least 90 min. The mouse in a chamber inhaled air gas for 30 min, and then hypoxic gas (7% O2 + 3% CO2 balanced in N2) for 20 min. Water but no food was provided at all times during these experiments. Body weights (BW) just before measurements of ventilation and metabolic rate were summarized during each dietary state in each genotype.
A separate animal group was used for fed and fasted states for the blood analysis, and sampling of blood started approximately at 1300 hours. The study protocol was approved by the Showa University Animal Experiments Committee.
Measurement of lung ventilation
Respiratory variables of conscious WT (n = 8) and H1RKO mice (n = 8) were measured with a whole-body, unrestrained, plethysmograph (WBP) (PLY3211; Buxco Electronics, Sharon, CT, USA), described elsewhere in detail [26]. Each mouse was measured for respiratory frequency (Rf, breaths/min), tidal volume (VT, ml BTPS/min, BTPS; body temperature and pressure, saturated with water vapor), minute ventilation (\( \dot{V}_{\text{E}} \), ml BTPS/min), and mean inspiratory flow (VT/TI, ml BTPS/s), which were recorded breath-by-breath throughout all baseline and experimental periods. VT and \( \dot{V}_{\text{E}} \) were normalized per 10 g of BW. Hypoxic gas contained 3% CO2 to compensate for the reduction in arterial PaCO2 caused by the “blowing off” of CO2 during elevated ventilation associated with hypoxia [27, 28].
Measurement of metabolic rate
WT mice (n = 7) and H1RKO mice (n = 8) were analyzed for aerobic metabolism. Collected gases from the inflow and outflow of an animal chamber (120 × 70 × 60 mm) were analyzed by automated mass spectrometry (ARCO-1000; ARCO System, Chiba, Japan). O2 consumption (\( \dot{V}{\text{O}}_{ 2} \), ml STPD/min; STPD, standard temperature and pressure, dry), CO2 excretion (\( \dot{V}{\text{CO}}_{2} \), ml STPD/min), and the respiratory exchange ratio (R) were recorded every minute and normalized per kilogram of body weight. Metabolic variables for resting state were evaluated from averaged 3-min values just before a start of hypoxic gas inhalation to ensure stability, and the variables after hypoxic gas inhalation were evaluated as average values every 1 min from 11 to 20 min. The unsettled variables after exchange of air for hypoxic gas were omitted from 1 to 10 min.
Glucose, lipid and related substances in the blood
Glucose and lactate levels of WT mice (n = 10) and H1RKO mice (n = 10) were measured in blood collected from the tail vein with the FreeStyle Blood Glucose Monitoring System (Kissei Pharmaceutical, Matsumoto, Japan) and Lactate Pro (Arkray, Kyoto, Japan), respectively. Ten fed WT and H1RKO mice, and 10 fasted WT and H1RKO mice were used.
Serum lipids and their metabolites were examined during the fed state (n = 10 in each genotype) and fasting state (n = 10 in WT, and n = 8 in H1RKO). Mice were anesthetized with an intraperitoneal injection of 50 mg/kg ketamine hydrochloride. Blood was collected from the right ventricle, and centrifuged to yield serum. Serum levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), free fatty acids (FFA), and ketone bodies were measured by methods described previously [14].
Statistical analysis
Data were expressed as mean ± SEM. To assess the effects of hypoxia at different fed/fasted states in different genotypes, a three-way analysis of variance (ANOVA) for repeated measures was performed for Rf, VT, \( \dot{V}_{\text{E}} \), mean inspiratory flow, and metabolic variables (\( \dot{V}{\text{O}}_{ 2} \), \( \dot{V}{\text{CO}}_{2} \), and R). Genotype was a between-group factor, while time and fed/fasted state factors were within-group factors. When an overall significant main effect or an interaction was found for a fed/fasted state, fed/fasted comparison of averaged values during 15–20 min was performed by the t test. Glucose and lactate levels in blood were analyzed by a three-way ANOVA with a repeated measurement. Genotype and the fed/fasted state were between-group factors, and the normoxia/hypoxia state was a within-group factor. Then, post hoc analysis was performed by the t test adjusted for multiple comparisons. Levels of lipid and related substances were analyzed by a two-way ANOVA. For these analyses, we used SPSS II software (SPSS Japan, Tokyo, Japan). Statistical significance was set at P < 0.05.
Results
Changes in ventilation
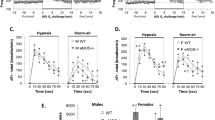
Figure 1a–h shows the ventilatory response pattern to hypoxia during fed/fasted states in each genotype. In fed WT mice, \( \dot{V}_{\text{E}} \) initially increased from 6.6 ± 0.4 ml/min/10 g BW to 29.3 ± 0.6 ml/min/10 g BW, at 5 min after the start of hypoxic gas exposure and then gradually declined (HVD) to 22.7 ± 1.9 ml/min/10 g BW at 20 min. The time course of \( \dot{V}_{\text{E}} \) was accompanied by changes in Rf, VT, and mean inspiratory flow in similar way (initial increase and sequential decline). In WT mice, the increase in Rf in the fasted state was lower than in the fed state (Fig. 1a; three-way ANOVA, main effect of fasting, P < 0.05) but the increase in VT in fasted WT mice exceeded the fed mice (Fig. 1b). The VT level was significantly higher in the fasted state than in the fed state throughout the period of hypoxic gas inhalation (three-way ANOVA, fasting × time × genotype interaction, P < 0.05), particularly during the 15–20 min period (t test, P < 0.05). Consequently, the initial increase in \( \dot{V}_{\text{E}} \) was unchanged by dietary states (Fig. 1c). The response of \( \dot{V}_{\text{E}} \) showed a minimal increase in the HVD during the fasted state, but the increase was not significant (t test). Mean inspiratory flow showed a similar profile for each dietary state as the \( \dot{V}_{\text{E}} \) profile (Fig. 1d). In fed H1RKO mice, the \( \dot{V}_{\text{E}} \), which was 5.8 ± 0.2 ml/min/10 g BW at rest had increased to 27.5 ± 2.5 ml/min/10 g BW at 5 min, and maintained this level during the 20-min test period (Fig. 1g). This indicates that HVD was blunted in the fed H1RKO mice. However, \( \dot{V}_{\text{E}} \) in the fasted H1RKO mice showed an initial increase and then a significant decline alongside the Rf patterns (Fig. 1e–g; three-way ANOVA, fasting × time × genotype interaction, P < 0.05). Mean inspiratory flow showed a similar profile for fed and fasted states as the \( \dot{V}_{\text{E}} \) profile (Fig. 1h, three-way ANOVA, fasting × time × genotype interaction, P < 0.05). The variables during the 15–20 min period in Rf, \( \dot{V}_{\text{E}} \) and mean inspiratory flow were lower in fasted states than in fed states (t test, P < 0.05). Statistical analysis supports a reversed fed/fasted state variation for VT, \( \dot{V}_{\text{E}} \), and mean inspiratory flow between WT and H1RKO mice.
Ventilatory responses to hypoxic gas exposure (7% O2 + 3% CO2 in N2) in wild-type (WT) mice (a–d) and histamine H1 receptor-knockout (H1RKO) mice (e–h). Respiratory frequency (Rf, a, e), tidal volume (VT, b, f), minute ventilation (\( \dot{V}_{\text{E}} \), c, g), and mean inspiratory flow (d, h). Open symbols indicate values during the fed state, and closed symbols indicate values during the fasted state. Statistical results of three-way ANOVA are indicated in (e–h). Significant differences between fed and fasted states were analyzed as mean values during a 15- to 20-min period (Student’s t test, vs fed, *P < 0.05)
Changes in metabolic rate
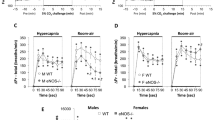
Figure 2a–f shows \( \dot{V}{\text{O}}_{ 2} \), \( \dot{V}{\text{CO}}_{2} \), and R values in response to hypoxia during the fed/fasted states of each genotype. In WT mice, hypoxia decreased \( \dot{V}{\text{O}}_{ 2} \) from 28.0 ± 1.8 at rest to 20.4 ± 2.5 ml/min/kg at 20 min in the fed state, but the decrease induced by hypoxia was slight in the fasted state (Fig. 2a, three-way ANOVA, main effect of time, P < 0.05, fasting × genotype interaction, P < 0.05). The \( \dot{V}{\text{O}}_{ 2} \) levels averaged during 15–20 min in the fasted state exceeded those in the fed state in WT mice (t test, P < 0.05). In WT mice, hypoxia increased \( \dot{V}{\text{CO}}_{2} \) levels from 22.6 ± 1.2 at rest to 25.7 ± 1.4 ml/min/kg BW at 11 min and recovered to 21.2 ± 1.4 ml/min/kg BW at 20 min in the fed state, with no differences from those in the fasted state (Fig. 2b; three-way ANOVA, main effect of time, P < 0.05). R values were increased from 0.81 ± 1.1 at rest to 1.0 ± 0.04 at 11 min in the fed state, but the levels of R value were low throughout the test period in the fasted state (Fig. 2c; three-way ANOVA, main effect of time, P < 0.05, main effect of fasting, P < 0.05). The R values during 15–20 min were significantly different between fed and fasted states (t test, P < 0.05). In H1RKO mice, \( \dot{V}{\text{O}}_{ 2} \) values were 26.5 ± 1.6 at rest and 25.8 ± 2.8 ml/min/kg at 20 min in the fed state, and the fed/fasted variation was reversed in comparison to those in WT mice (Fig. 2d; three-way ANOVA, fasting × genotype interaction, P < 0.05). The \( \dot{V}{\text{O}}_{ 2} \) levels during 15–20 min in the fasted state were lower than those in the fed state (t test, P < 0.05). \( \dot{V}{\text{CO}}_{2} \) levels increased from 21.4 ± 0.7 ml/min/kg BW at rest to 26.9 ± 1.3 ml/min/kg BW at 11 min and then declined to 24 ± 1.6 ml/min/kg BW at 20 min in the fed state, and the levels were lower throughout the test period in the fasted state (Fig. 2e; three-way ANOVA, main effect of time, P < 0.05, fasting × genotype interaction, P < 0.05). The values during 15–20 min were significantly different between dietary conditions (t test, P < 0.05). Hypoxia increased R values from 0.81 ± 0.04 at rest to 1.0 ± 0.06 at 11 min and were maintained during the test period (three-way ANOVA, main effect of time, P < 0.05), but the fed/fasted variation was minimal compared to WT mice (Fig. 2f, main effect of fasting, P < 0.05).
Metabolic responses to hypoxic gas exposure (7% O2 + 3% CO2 in N2) in wild-type (WT) mice (a–c) and histamine H1 receptor-knockout (H1RKO) mice (d–f). Metabolic variables (\( \dot{V}{\text{O}}_{ 2} \), \( \dot{V}{\text{CO}}_{2} \), and R). Open symbols indicate values during the fed state, and closed symbols indicate values during the fasted state. Results from three-way ANOVA are indicated in (d–f). Fed/fasted comparison of averaged values during 15–20 min is indicated (t test, *P < 0.05)
Body weight
The body weights of mice used for the measurement of ventilation and metabolic rate were 29.96 ± 0.53 g during a fed state and 29.06 ± 0.58 g during a fasted state in WT mice (n = 15, paired t test, P < 0.05), and 29.69 ± 0.94 g during a fed state and 29.16 ± 0.88 g during a fasted state in H1RKO mice (n = 16).
Relationship between \( \dot{V}_{\text{E}} \) and \( \dot{V}{\text{O}}_{ 2} \), \( \dot{V}{\text{CO}}_{2} \)
Averaged values every 1 min of \( \dot{V}_{\text{E}} \) and \( \dot{V}{\text{O}}_{ 2} \) during 11–20 min are plotted in Fig. 3a–d to evaluate the relationship between ventilation and metabolic rate during HVD. In WT mice, the plot area of \( \dot{V}_{\text{E}} /\dot{V}{\text{O}}_{ 2} \) in the fasted group was extended diagonally to the upper right of the plots for the fed group (Fig. 3a). The \( \dot{V}_{\text{E}} \) increase in the fasted mice was in accord with the \( \dot{V}{\text{O}}_{ 2} \) increase. The plot area of \( \dot{V}_{\text{E}} /\dot{V}{\text{CO}}_{2} \) for the fasted mice had a minimal shift upwards (Fig. 3b). In H1RKO mice, the plot area of \( \dot{V}_{\text{E}} /\dot{V}{\text{O}}_{ 2} \) for the fasted group was below the fed group (Fig. 3c). The plot area of \( \dot{V}_{\text{E}} /\dot{V}{\text{CO}}_{2} \) for the fasted group was below and to the left of the fed group (Fig. 3d). The effect of fasting on \( \dot{V}_{\text{E}} /\dot{V}{\text{O}}_{ 2} \) and \( \dot{V}_{\text{E}} /\dot{V}{\text{CO}}_{2} \) showed a different profile between genotypes.
Averaged values every minute are plotted over a 11- to 20-min period after hypoxic gas exposure. Relationship between \( \dot{V}_{\text{E}} \) and \( \dot{V}{\text{O}}_{ 2} \) (a, c) and \( \dot{V}_{\text{E}} \) and \( \dot{V}{\text{CO}}_{2} \) (b, d). Distribution areas of variables in the fed (open symbols) and fasted (closed symbols) states are surrounded by circles. Note the relationship of the position in each circle
Changes in glucose and lactate concentrations in the blood
Table 1 shows that the glucose concentration was decreased by fasting in both genotypes (three-way ANOVA, main effect of food, P < 0.05, t test, vs fed, P < 0.025), and increased by hypoxia (time, main effect of time, P < 0.05). In addition, a three-way ANOVA showed a time × genotype interaction (P < 0.05), suggesting that the increase in glucose concentration during hypoxia was more significant in H1RKO mice than in WT mice. Table 1 also shows that the lactate concentration significantly increased during hypoxia (a three-way ANOVA, a main effect of time, P < 0.05). The lactate concentration was not different between dietary states or genotypes.
Changes in lipid and related substances in the blood
Table 2 shows that fasting increased FFA and ketone bodies in both genotypes (two-way ANOVA, main effects of fasting for FFA and ketone bodies, P < 0.05). The increase in the concentration of ketone bodies was more extensive in H1RKO than WT mice (fasting × genotype interaction for ketone bodies, P < 0.05). TG levels in WT mice were higher than those in H1RKO mice. There was a significant main effect of genotype on TG (P < 0.05). Neither of the cholesterol subtypes was significantly different between dietary states or genotypes.
Discussion
Major findings of the present study were that fasting altered the breathing pattern of the biphasic ventilatory responses to acute hypoxic gas exposure, particularly in the HVD phase, and that histamine H1 receptors were involved in these responses in both fed and fasted conditions. Fasting lowered R values and blood glucose concentrations, and increased blood levels of FFA and ketone bodies, showing the predominance of lipid metabolism. Changes in energy metabolism induced by fasting influence the hypoxic ventilatory response.
Fed/fasted variation in ventilatory and metabolic responses to hypoxia
Acute exposure to hypoxic gas affected \( \dot{V}_{\text{E}} \) and \( \dot{V}{\text{O}}_{ 2} \) with some differences between fed and fasted states. A fed/fasted variation was observed in the HVD phase rather than an initial increase phase in the biphasic ventilatory response to hypoxia (Fig. 1). The biphasic profile of ventilatory response to hypoxia is determined by a balance between stimulatory and inhibitory inputs to the medullary respiratory center [6]. The stimulatory input is activated by the peripheral chemoreceptors, whose afferent activity does not adapt to the hypoxic condition within 2–3 h [5, 29]. The inhibitory input is initiated by alkalosis followed by hyperventilation and an increase in cerebral blood flow, hypometabolism, or central neural depression [1, 6]. Honda et al. [30] showed that the stimulatory and inhibitory inputs come from separate mechanisms, because hypoxic hyperventilation followed by O2 breathing augmented the hypoxic ventilatory response without affecting the biphasic ventilatory decline.
In the present study, the initial increase in \( \dot{V}_{\text{E}} \) was not affected by fasting, although there are some differences in breathing pattern between dietary states. In addition, mean inspiratory flow, which is an index of inspiratory motor drive as an output of the respiratory center [31], was not altered during the initial phase. These results suggest that, in general, peripheral chemoreceptor activity does not participate in the fed/fasted variation of the central respiratory output. This is not necessarily consistent with previous notions that hypoglycemia stimulates chemoafferent activity, or that it increases \( \dot{V}_{\text{E}} \) and ventilatory responses to hypoxia or hypercapnia [32–34]. The carotid bodies are believed to be important sensors for hypoxia and hypoglycemia, whose action is based on an insulin-induced severe hypoglycemia [35]. However, by using carotid body chemoreceptor cells, Conde et al. [36] demonstrated that hypoxia-evoked release of catecholamine and hypoxia-induced action potentials of the carotid sinus nerve were identical under normal and low glucose concentrations. It is still questioned whether or not low glucose stimulates the carotid body chemoreceptor [36]. Our 12-h fasting treatment decreased the glucose concentration to 75% of the normal level. Our results under mild hypoglycemia did not support the upregulation in the \( \dot{V}_{\text{E}} \) response to hypoxia mediated by peripheral chemoreceptors.
In the HVD phase, the fasting effect on \( \dot{V}_{\text{E}} \) was not significant despite a marked increase in VT in WT mice. However, the fasting effect on \( \dot{V}{\text{O}}_{ 2} \) was significant in that phase, suggesting that hypometabolism was reduced (Fig. 2a). In addition, Fig. 3a shows that \( \dot{V}_{\text{E}} \) changes along with \( \dot{V}{\text{O}}_{ 2} \) in these states. We focused the fasting effect on ventilation in relation to metabolism. Fasting lowered the R values and blood glucose concentration, and increased FFA and ketone bodies, showing a tendency toward lipid metabolism. Fatty-acid metabolism, namely β-oxidation through the mitochondrial oxidative pathway, seemed to require an increase in \( \dot{V}{\text{O}}_{ 2} \) compared to glycolytic metabolism during hypoxia. That is to say, HVD could be modulated by adequate stimuli based on dominant lipid metabolism.
During hypoxia, as the oxygen level decreases, ATP generation shifts from the mitochondrial oxidative phosphorylation pathway to the cytoplasmic oxygen independent pathway of glycolysis. Severe hypoxia induces an increase in anaerobic glucose utilization in the cytoplasm (reviewed by Mortola et al. [37]). Hypoxia in the present study was severe, because the PaO2 has been measured previously as 33.8 mmHg during inhalation of the same type of hypoxic gas [7]. Indeed, an increase in the serum lactate concentration was observed as a product of anaerobic metabolism. However, lactate accumulation occurred in the same range in both the fed and fasted states during hypoxia (Table 1), showing that the progression of anaerobic metabolism was unchanged. Yoshino et al. [38] suggested that energy metabolism during hypoxia depends on glycolysis utilizing blood glucose as the substrate in fed rats, and depends on the glycogen degradation in the liver through adrenergic pathways in fasted rats. Glycogen degradation is important to provide the caloric needs in the fasted state, and this form of energy metabolism might contribute to the decrease in hypometabolism and modulation of breathing pattern during HVD.
Metabolic acidosis might occur because of an increase in ketone bodies in the fasted mice, and the ventilatory control of the acid–base status might modulate the ventilatory pattern during HVD. However, the \( \dot{V}_{\text{E}} /\dot{V}{\text{O}}_{ 2} \) ratio and the \( \dot{V}_{\text{E}} /\dot{V}{\text{CO}}_{2} \) ratio were the same in the fed and fasted states, suggesting that the hyperpnoeic level of HVD is possibly adequate for the metabolic rate in the fasted state. Blood gas analysis during inhalation of 7% O2 + 3% CO2 has shown that pH, PaCO2 and [HCO3 −] are normal in ad libitum fed mice [14]. Further analysis may be needed to determine the degree of metabolic acidosis, although the operation would be extremely invasive to the fasting mice.
Genotype variations in ventilatory and metabolic responses to hypoxia
Remarkable genotype variations were observed in the fasting effects on the hypoxic ventilatory responses (Fig. 1). The fed H1RKO mice showed decreases in HVD and hypometabolism, which is consistent with our previous study. We suggested previously that H1 receptors contribute to the changes in the metabolic process during hypoxia and the development of HVD. H1RKO mice showed a blunted HVD that is adequately controlled according to metabolic demand [7].
Hypoxic ventilatory decline reduces the metabolic demand and limits O2 demand at the organism level, which is important for hypoxia-adaptive mechanisms [6]. The decrease in HVD is unfavorable for adaptation to the hypoxic condition and energy efficiency. In the present study, \( \dot{V}_{\text{E}} \) during the HVD phase was slightly moved in accord with the \( \dot{V}{\text{O}}_{ 2} \) increase under the predominant lipid metabolism in the fasted WT mice (Fig. 3a). In turn, \( \dot{V}_{\text{E}} \) during the HVD was significantly shifted in accord with the \( \dot{V}{\text{CO}}_{2} \) decrease in the fasted H1RKO mice (Fig. 3d). This genotype difference is likely due to the impairment of lipolysis, because hypothalamic histamine has a characteristic of accelerating lipolysis through the sympathetic pathway via H1 receptors [22, 23, 39]. It has been demonstrated that central administration of histamine or an H3 receptor antagonist, thioperamide, increases the lipolytic response in white adipose tissue of rats [23]. H1RKO mice have also shown an increase in food intake and visceral adiposity, which is accompanied by a decrease in energy expenditure [40]. In the present H1RKO mice, the shift in the R value induced by fasting was minimal in comparison to WT mice. That is to say, the conversion to lipid metabolism in response to fasting is low. Impaired lipid metabolism is also supported by the low TG concentrations in H1RKO mice (Table 2). During starvation, TG is broken down into FFAs, which are released into the circulation and supply other tissues with energy [41]. The low TG concentration in H1RKO mice indicates a deficit in substrate for energy metabolism during the fasted state. Thus, the fasted condition emphasizes the contribution of H1 receptors to lipid metabolism, implicating H1 receptors and lipid metabolism as novel factors in the decrease in HVD during fasted states.
We previously studied the metabolic rate throughout a 24-h measurement period under normoxic conditions. H1RKO mice show higher R values than WT mice, suggesting a predominance of glycolytic metabolism [42]. This metabolic trait was thought to be advantageous for anaerobic metabolism during hypoxia. However, the present results did not show genotype differences for the R values during hypoxia, suggesting that glycolytic metabolism during hypoxia was not necessarily predominant in H1RKO mice in comparison to WT mice. Moreover, there was no genotype difference in the lactate concentration during hypoxia (Table 1). Lactate is a product of anaerobic metabolism and an important intermediary in metabolic processes as a mobile fuel [43]. After all, the predominant glycolytic metabolism observed during normoxia in H1RKO mice is not necessarily advantageous for anaerobic metabolism during hypoxia.
The effects of the brain histamine have been demonstrated in glucose metabolism. Hypoglycemic stress increases histamine release, which controls energy supply in the brain via H1 receptors [44], and glucoprivation increases hypothalamic histamine turnover [45]. Furthermore, hypothalamic histamines cause systemic hyperglycemia via catecholamine release from the adrenal medulla [21]. The evidence suggests that histamine can rescue animals from starvation by controlling glucose levels. In the present study, the glucose concentration was different between genotypes in interaction with time (hypoxia). The difference was produced by a slightly lower concentration in H1RKO mice than in WT mice during normoxia in each dietary condition. Glucose levels are influenced in their regulation and/or utilization during normoxia by H1 receptors.
In conclusion, we found that 12-h fasting changed the ventilatory response pattern to hypoxia in the HVD phase with a marked increase in VT. Fasting seemed to increase \( \dot{V}{\text{O}}_{ 2} \) due to a predominance of lipid metabolism and/or glycogen degradation for caloric needs. H1RKO mice had a blunted HVD in the fed state, but probably produced HVD due to moderation of the dominant lipid metabolism in the fasted state. Histamine H1 receptors are involved in the HVD phase in both fed and fasted states through control of lipid and glucose metabolisms. Histamine H1 receptors are essential for adaptation to the change in environmental conditions.
References
Mortola JP (1996) Ventilatory responses to hypoxia in mammals. In: Haddad GG, Lister G (eds) Tissue oxygen deprivation. Marcel Dekker, New York
Zwillich CW, Sahn SA, Weil JV (1977) Effects of hypermetabolism on ventilation and chemosensitivity. J Clin Invest 60:900–906
Weil JV, Byrne-Quinn E, Sodal IE, Kline JS, McCullough RE, Filley GF (1972) Augmentation of chemosensitivity during mild exercise in normal man. J Appl Physiol 33:813–819
Doekel RC Jr, Zwillich CW, Scoggin CH, Kryger M, Weil JV (1976) Clinical semi-starvation: depression of hypoxic ventilatory response. N Engl J Med 295:358–361
Vizek M, Pickett CK, Weil JV (1987) Biphasic ventilatory response of adult cats to sustained hypoxia has central origin. J Appl Physiol 63:1658–1664
Neubauer JA, Melton JE, Edelman NH (1990) Modulation of respiration during brain hypoxia. J Appl Physiol 68:441–451
Ishiguro T, Iwase M, Kanamaru M, Izumizaki M, Ohshima Y, Homma I (2006) Impaired ventilation and metabolism response to hypoxia in histamine H1 receptor-knockout mice. Respir Physiol Neurobiol 154:331–341
Ericson H, Blomqvist A, Kohler C (1991) Origin of neuronal inputs to the region of the tuberomammillary nucleus of the rat brain. J Comp Neurol 311:45–64
Inagaki N, Yamatodani A, Ando-Yamamoto M, Tohyama M, Watanabe T, Wada H (1988) Organization of histaminergic fibers in the rat brain. J Comp Neurol 273:283–300
Iwase M, Homma I, Shioda S, Nakai Y (1993) Histamine immunoreactive neurons in the brain stem of the rabbit. Brain Res Bull 32:267–272
Izumizaki M, Iwase M, Kimura H, Kuriyama T, Homma I (2000) Central histamine contributed to temperature-induced polypnea in mice. J Appl Physiol 89:770–776
Kanamaru M, Iwase M, Homma I (2001) Neuronal histamine release elicited by hyperthermia mediates tracheal dilation and pressor response. Am J Physiol Regul Integr Comp Physiol 280:R1748–R1754
Miyamoto K, Iwase M, Kimura H, Homma I (2004) Central histamine contributes to the inspiratory off-switch mechanism via H1 receptors in mice. Respir Physiol Neurobiol 144:25–33
Ohshima Y, Iwase M, Izumizaki M, Ishiguro T, Kanamaru M, Nakayama H, Gejyo F, Homma I (2007) Hypoxic ventilatory response during light and dark periods and the involvement of histamine H1 receptor in mice. Am J Physiol Regul Integr Comp Physiol 293:R1350–R1356
Iwase M, Izumizaki M, Miyamoto K, Ishiguro T, Kanamaru M, Homma I (2007) Lack of histamine type-1 receptors impairs the thermal response of respiration during hypoxia in mice (Mus musculus). Comp Biochem Physiol A Mol Integr Physiol 146:242–251
Itowi N, Nagai K, Nakagawa H, Watanabe T, Wada H (1988) Changes in the feeding behavior of rats elicited by histamine infusion. Physiol Behav 44:221–226
Fukagawa K, Sakata T, Shiraishi T, Yoshimatsu H, Fujimoto K, Ookuma K, Wada H (1989) Neuronal histamine modulates feeding behavior through H1-receptor in rat hypothalamus. Am J Physiol 256:R605–R611
Green MD, Cox B, Lomax P (1976) Sites and mechanisms of action of histamine in the central thermoregulatory pathways of the rat. Neuropharmacology 15:321–324
Tsai CL, Matsumura K, Nakayama T, Itowi N, Yamatodani A, Wada H (1989) Effects of histamine on thermosensitive neurons in rat preoptic slice preparations. Neurosci Lett 102:297–302
Masaki T, Yoshimatsu H (2006) The hypothalamic H1 receptor: a novel therapeutic target for disrupting diurnal feeding rhythm and obesity. Trends Pharmacol Sci 27:279–284
Nishibori M, Itoh Y, Oishi R, Saeki K (1987) Mechanism of the central hyperglycemic action of histamine in mice. J Pharmacol Exp Ther 241:582–586
Masaki T, Yoshimatsu H, Chiba S, Watanabe T, Sakata T (2001) Central infusion of histamine reduces fat accumulation and upregulates UCP family in leptin-resistant obese mice. Diabetes 50:376–384
Tsuda K, Yoshimatsu H, Niijima A, Chiba S, Okeda T, Sakata T (2002) Hypothalamic histamine neurons activate lipolysis in rat adipose tissue. Exp Biol Med (Maywood) 227:208–213
Finn PF, Dice JF (2006) Proteolytic and lipolytic responses to starvation. Nutrition 22:830–844
Miyamoto K, Iwase M, Nyui M, Arata S, Sakai Y, Gabazza EC, Kimura H, Homma I (2006) Histamine type 1 receptor deficiency reduces airway inflammation in a murine asthma model. Int Arch Allergy Immunol 140:215–222
Izumizaki M, Tamaki M, Suzuki Y, Iwase M, Shirasawa T, Kimura H, Homma I (2003) The affinity of hemoglobin for oxygen affects ventilatory responses in mutant mice with Presbyterian hemoglobinopathy. Am J Physiol Regul Integr Comp Physiol 285:R747–R753
Pepelko WE, Dixon GA (1975) Arterial blood gases in conscious rats exposed to hypoxia, hypercapnia, or both. J Appl Physiol 38:581–587
Iwase M, Izumizaki M, Kanamaru M, Homma I (2004) Effects of hyperthermia on ventilation and metabolism during hypoxia in conscious mice. Jpn J Physiol 54:53–59
Barnard P, Andronikou S, Pokorski M, Smatresk N, Mokashi A, Lahiri S (1987) Time-dependent effect of hypoxia on carotid body chemosensory function. J Appl Physiol 63:685–691
Honda Y, Tani H, Masuda A, Kobayashi T, Nishino T, Kimura H, Masuyama S, Kuriyama T (1996) Effect of prior O2 breathing on ventilatory response to sustained isocapnic hypoxia in adult humans. J Appl Physiol 81:1627–1632
Milic-Emili J, Grunstein MM (1976) Drive and timing components of ventilation. Chest 70:131–133
Kumar P, Bin-Jaliah I (2007) Adequate stimuli of the carotid body: more than an oxygen sensor? Respir Physiol Neurobiol 157:12–21
Zhang M, Buttigieg J, Nurse CA (2007) Neurotransmitter mechanisms mediating low-glucose signalling in cocultures and fresh tissue slices of rat carotid body. J Physiol 578:735–750
Bin-Jaliah I, Maskell PD, Kumar P (2005) Carbon dioxide sensitivity during hypoglycaemia-induced, elevated metabolism in the anaesthetized rat. J Physiol 563:883–893
Bin-Jaliah I, Maskell PD, Kumar P (2004) Indirect sensing of insulin-induced hypoglycaemia by the carotid body in the rat. J Physiol 556:255–266
Conde SV, Obeso A, Gonzalez C (2007) Low glucose effects on rat carotid body chemoreceptor cells’ secretory responses and action potential frequency in the carotid sinus nerve. J Physiol 585:721–730
Mortola JP, Matsuoka T, Saiki C, Naso L (1994) Metabolism and ventilation in hypoxic rats: effect of body mass. Respir Physiol 97:225–234
Yoshino M, Murakami K, Katsumata Y, Takabayashi A, Mori S (1987) Stimulation of anaerobic metabolism in rats at high altitude hypoxia—adrenergic effects dependent on dietary states. Comp Biochem Physiol B 88:651–654
Masaki T, Yoshimatsu H, Chiba S, Watanabe T, Sakata T (2001) Targeted disruption of histamine H1-receptor attenuates regulatory effects of leptin on feeding, adiposity, and UCP family in mice. Diabetes 50:385–391
Masaki T, Chiba S, Yasuda T, Noguchi H, Kakuma T, Watanabe T, Sakata T, Yoshimatsu H (2004) Involvement of hypothalamic histamine H1 receptor in the regulation of feeding rhythm and obesity. Diabetes 53:2250–2260
Zechner R, Strauss JG, Haemmerle G, Lass A, Zimmermann R (2005) Lipolysis: pathway under construction. Curr Opin Lipidol 16:333–340
Ishiguro T, Iwase M, Kanamaru M, Izumizaki M, Ohshima Y, Homma I (2006) Contribution of histamine type-1 receptor to metabolic and behavioral control of ventilation. J Physiol Sci 56:287–295
Gladden LB (2004) Lactate metabolism: a new paradigm for the third millennium. J Physiol 558:5–30
Sakata T, Kurokawa M, Oohara A, Yoshimatsu H (1994) A physiological role of brain histamine during energy deficiency. Brain Res Bull 35:135–139
Oohara A, Yoshimatsu H, Kurokawa M, Oishi R, Saeki K, Sakata T (1994) Neuronal glucoprivation enhances hypothalamic histamine turnover in rats. J Neurochem 63:677–682
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ohshima, Y., Iwase, M., Izumizaki, M. et al. Effects of fasting on hypoxic ventilatory responses and the contribution of histamine H1 receptors in mice. J Physiol Sci 61, 73–82 (2011). https://doi.org/10.1007/s12576-010-0124-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-010-0124-9