Abstract
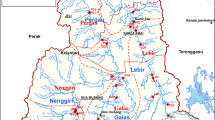
A study of water quality was conducted for Newton Creek that flows in an urban area in the city of Superior and discharges into Hog Island Inlet (HII). Water quality data were collected from five locations located along the creek flow from its head to its mouth. Throughout the year there was a wide range in measured values for the temperature, dissolved oxygen (DO), electro conductivity (EC), and pH. These ranges showed there were numerous factors affecting these properties of the creek, with the impact from oil refinery being the greatest. Some of the recorded salinity was observed to be much greater than the acute criteria. The high salinity of the creek water is attributed to the chloride and sodium concentrations with relatively elevated concentrations of sulfate and nitrate. A geochemical model revealed that the water chemical composition of HII was the result of a mixing ratio of about 90 % of Lake Superior water and 10 % of Newton Creek water. Faxon Creek, which was monitored for comparison purposes, recorded salinity concentrations much higher than the salinity of Newton Creek. Both Newton and Faxon creeks revealed different chemical makeup. Faxon Creek revealed that all major cation and anion concentrations in its water were higher than in Newton Creek waters with the exception of potassium, sulfate and nitrate. Nitrate and phosphorous concentrations in Newton Creek are much higher than in Faxon Creek and all the surface water in the area, and they exceed the recommended concentration values set by U.S. Environmental Protection Agency (EPA).
The chemical makeup and the variation of the major ions in both creeks show that the source of water contamination in Newton Creek originates mainly from the discharge of oil refinery treated wastewater with minimum contribution from surface runoff. The source of contamination and mainly the elevated salinity in Faxon Creek water is mainly from urban surface runoff from the city of Superior.











Similar content being viewed by others
References
Back W (1961) Techniques for mapping of hydrochemical facies. US Geol Surv Prof Pap 424D:380–382
Bajjali W, Johnson DR, Sandstorm NR (2007) Water quality assessment of Newton Creek of Superior, Wisconsin. In: 8th Annual university of Wisconsin system symposium for undergraduate research and creative activity, University of Wisconsin-Stout, April
Belser LW (1984) Bicarbonate uptake by nitrifiers: effects of growth rate, pH, substrate concentration, and metabolic inhibitors. Appl Environ Microbiol 48(6):1100–1104
Berounsky VM, Nixon SW (1993) Rates of nitrification along an estuarine gradient in Narragansett Bay. Estuar Coast 16(4):718–730. doi:10.2307/1352430
Burgess JE, Parsons SA, Stuetz RM (2001) Developments in odor control and waste gas treatment biotechnology: a review. Biotechnol Adv 19:35–63
DNR (1995) Newton Creek system sediment contamination site characterization report. Wisconsin Department of Natural Resources, December
Douglas County Land Conservation Committee (DCLCC) and Land Water Conservation Department (LWCD) (2009) Land and water resources management plan. Douglas County, WI
EPA (1993) Report to Congress on hydrogen sulfide air emissions associated with the extraction of oil and natural Gas. EPA-453/R-93-045
EPA (2000a) Aquatic life criteria for dissolved oxygen, fact sheet. Office of Science and Technology, Health and Ecological Criteria Division, Washington
EPA (2000b) Ambient water quality criteria recommendations-rivers and streams. USEPA, Washington
EPA (2008) Areas of Concern (AOCs) in the Great Lakes region http://www.greatlakes.net/envt/pollution/aoc.html#overview
EPA (2009) National primary drinking water regulation. EPA 816-F-09-004, USA
Feth JH (1981) Chloride in natural continental water—a review: U.S. Geological Survey Water Supply Paper 2176, p 30
Freeze RA, Cherry JA (1979) Groundwater. Prentice-Hall, Englewood Cliffs 07632
Gujer W, Jenkins D (1974) A nitrification model for contact stabilization activated sludge process. Water Res 9(5):5
Heathwaite AL (1997) Sources and pathways of phosphorus loss from agriculture. In: Tunny H, Carton OT, Brookes PC, Johnston AE (eds) Phosphorus loss from soil to water. CAB International, Wallingford, pp 205–223
Hem JD (1982) Conductance: a collective measure of dissolved ions. In: Minear RA, Keith LH (eds) Water analysis, vol 1. Inorganic species. Academic Press, New York, pp 137–161
Fang H-Y, Chou M-S, Huang C-W (1993) Nitrification of ammonia-nitrogen in refinery wastewater. Water Res 27(12):1761–1765
Kaushal SS, Groffman PM, Likens GE, Belt KT, Stack WP, Kelly VR, Band LE, Fisher GT (2005) Increased salinization of fresh water in the Northeastern United Stated. Proc Natl Acad Sci USA 102:13517–13520
Langmuir D (1997) Aqueous environmental chemistry. Prentice-Hall, Englewood Cliffs, p 600
Mackie GL (2001) Applied aquatic ecosystem concepts. Kendall/Hunt, Dubuque, 744 pp
Mainstone CP, Parr W (2002) Phosphorus in rivers—ecology and management. Sci Total Environ 282–283:25–47
Marsalek J (2003) Road salts in urban stormwater: an emerging issue in stormwater management in cold climates. Water Sci Technol 48:61–70
Merill DT, Manzione MA, Peterson JJ, Parker DS, Chow W, Hobbs AD (1986) Field evaluation of arsenic and selenium removal by iron coprecipitation. J-Water Pollut Control Fed 58:18–26
Merryfield N, Epstein E, Galvin A, Judziewicz E, Smith W (2000) A data compilation and assessment of coastal wetlands of Wisconsin’s Great Lake. Natural heritage inventory program of endangered resources. DNR, Madison, p 139
Meyer BW (1994) Changes in land use and land cover: a global perspective. Cambridge University Press, Cambridge, 537 pp
Mullaney JR, Lorenz DL, Arntson AD (2009) Chloride in groundwater and surface water in areas underlain by the glacial aquifer system, Northern United States: USGS, Scientific Investigations Report 2009–5086, p 41
Novotny EV, Murphy D, Stefan HG (2007) Salty lakes in Minnesota. Minneapolis, Minnesota: St. Anthony Falls laboratory. University of Minnesota; Project Report No 505
Olivera F, Seann R, Maidment D (1998) HEC-PrePro v. 2.0: An ArcView pre-processor for HEC’s hydrologic modeling system. In: 1998 ESRI User Conference, San Diego, CA
Parr W, Mainstone CP, Morgan N (1998) Nutrient budget for the upper reaches of the Hampshire Avon. Report to English Nature and the Environment Agency. WRc, Medmenham
Peters EN (1991) Chloride cycling in two forested lake watersheds in the west-central Adirondack mountains, New York, U.S.A. Water, Air, & Soil Pollution Focus 59:201–215
Ramstack JM, Fritz SC, Engstrom DR (2004) Twentieth-century water quality trends in Minnesota Lakes compared with pre-settlement variability. Can J Fish Aquat Sci 61:561–576
Salisbury FB, Ross CW (1978) Plant physiology. Wadsworth Publishing, Belmont, 422 pp
Scearce SN, Benninger RW, Weber AS, Sherrard SH (1980) Prediction of alkalinity changes in the activated sludge process. J -Water Pollut Control Fed 52:399–405
Shalaby HM (2006) Refining of Kuwait’s heavy crude oil: materials challenges. In: Workshop on corrosion and protection of metals. Arab school for science and technology, 3–7 December 2006, Kuwait
Acknowledgements
The author is grateful to the Douglas County Land and Water Conservation Department for funding the project. I also extend my great thanks for all my students who participate in sampling and analyzing the water.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bajjali, W. Water Quality Assessment of Newton Creek and Its Effect on Hog Island Inlet of Lake Superior. Water Qual Expo Health 4, 123–135 (2012). https://doi.org/10.1007/s12403-012-0071-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-012-0071-1