Abstract
Herpes zoster (HZ) is a common, painful and debilitating disease caused by the reactivation of latent varicella-zoster virus in ganglia. This clinical event occurs more frequently in the elderly and those who are immunocompromised. The most common complication of HZ is post-herpetic neuralgia (PHN) which is responsible for the highest HZ-related burden of illness and is challenging to treat. Due to the important clinical and economic impact of HZ and PHN, and the suboptimal treatments that are currently available, HZ vaccination is an important approach to reduce the burden of illness. Currently, one-dose, live-attenuated vaccine is licensed in the United States and Europe to prevent HZ and it is included in some national immunization programs. The clinical efficacy, safety and tolerability of the vaccine has been demonstrated in two large phase III clinical trials, involving more than 38,000 and 22,000 individuals aged ≥60 and 50–59 years, respectively. This comprehensive review summarizes the extensive “real-world” effectiveness and safety data from both immunocompetent and immunocompromised individuals. These data confirm those from the clinical trials, supporting the use of HZ vaccine in clinical practice and provide evidence that the current recommendations for immunocompromised individuals should be revised.
Funding: Funding for the editorial assistance, article processing charges, and open access fee for this publication was provided by Sanofi Pasteur MSD.
Similar content being viewed by others
Introduction
The varicella-zoster virus (VZV) is a DNA virus that belongs to the Herpesviridae family, subfamily Alphaherpesvirinae [1, 2]. After primary infection with VZV, it remains dormant in the dorsal root or cranial nerve sensory ganglia. When reactivation occurs it causes herpes zoster (HZ), also known as shingles, usually characterized by a vesicular eruption with severe pain in a single dermatome segment [2–5].
The course of acute HZ episodes can be complicated by post-herpetic neuralgia (PHN), which is a painful and debilitating condition defined as a chronic neuropathic resilient pain that persists for 3 months or more from the initial onset of the rash [6, 7]. The risk of VZV reactivation increases with the decline of cell-mediated immunity, particularly due to the aging [3]. In addition, individuals who are immunocompromised due by certain diseases, such as hematologic malignancies and solid cancers have an increased risk for a higher incidence and severity of HZ and its neurologic and ophthalmologic complications and visceral dissemination [8–16]. The risk of HZ and its complications is also higher in patients with autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and Crohn’s disease due to both the pathologic process and to their immunosuppressive treatments [17–24].
The annual incidence rates of HZ are similar across countries, ranging from 3 to 5 per 1000 people in North America, Europe and Asia–Pacific [25]. In all countries, HZ shows a similar age-specific epidemiologic pattern, with a steep increase in the incidence rate in those aged 50 years or more, with up to 8–12 HZ cases per 1000 persons in those aged 80 years or more [25]. Nearly 50% of individuals aged 85 years or more have experienced HZ [3].
The incidence of PHN also increases with age, ranging between 5% and about 30% in the adult population and between 25% and 50% in adults aged 50 years or more [25].
Herpes zoster and PHN affect the health-related quality of life and induce a substantial economic burden [26]. PHN has been reported to have an impact on physical, psychological, functional and social aspects of patients’ lives causing interference with daily activities. HZ and PHN cannot be managed satisfactorily with the currently available treatments and severe cases require extensive healthcare resources, particularly for specialist consultations, diagnostic examinations, and sometimes, hospitalization [27].
The live-attenuated HZ vaccine, Zostavax® (Merck Sharp & Dohme Corporation), was first licensed by the American Food and Drug Administration (FDA) in 2006 for the prevention of HZ in adults aged ≥60 years [28]. In the same year, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) authorized Zostavax for the prevention of both HZ and PHN [29]. In March 2011, the FDA extended the indication to include adults aged between 50 and 59 years [30]. Zostavax is currently the only HZ vaccine approved for use in the United States (US) and in Europe [29, 31]. After its licensure in the US, the Advisory Committee on Immunization Practices (ACIP) recommends the use of Zostavax for prevention of HZ and its complications among adults aged ≥60 years [32]. In this age group the vaccine is reimbursed by private health insurance or through public program.
Advisory Committee on Immunization Practices also considered the use of HZ vaccine among adults aged 50 through 59 years [30], but up to date declines to recommend the vaccine in this age group, citing shortages of Zostavax and limited data on long-term protection in this age group.
In Europe, Zostavax has been recommended and public funded in many countries, such as United Kingdom, Greece, France and some regions in Germany and Italy, according to different immunization strategies. In Austria, the HZ vaccine is recommended to adults aged ≥50 years, but it is not public funded. The results from two phase III clinical trials, which lead to licensure of the vaccine, demonstrated that Zostavax was safe, well-tolerated and effective in preventing HZ and PHN in adults aged ≥50 years [33, 34]. The efficacy of Zostavax was reported to be 51.3% [95% confidence interval (CI) 44.2; 57.6] and 66.5% (95% CI 47.5; 79.2) for preventing HZ and PHN, respectively, in adults aged ≥60 years in shingles prevention study (SPS; ClinicalTrials.gov identifier: NCT00007501) [32]. In the Zostavax Efficacy and Safety Trial (ZEST; ClinicalTrials.gov identifier: NCT00534248), its efficacy was 69.8% (95% CI 54.1; 80.6) for preventing HZ in adults aged 50–59 years [34].
Since the introduction of Zostavax in the US immunization program nearly 10 years ago, several effectiveness studies assessing the efficacy of HZ vaccine in a ‘real-life’ setting have been conducted in immunocompetent and immunocompromised individuals. Here we will summarize the results obtained in these effectiveness studies. This article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
HZ Vaccine Effectiveness in Immunocompetent Subjects
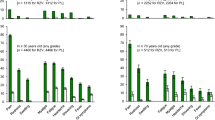
Since licensure, the effectiveness of HZ vaccine has been assessed in three retrospective studies in immunocompetent subjects in the US (Table 1) [35–37]. One of these analyzed data for 303,044 enrollees from the Kaiser Permanente Southern California (KPSC) health plan from January 2007 to December 2009 [35]. The results showed that the HZ vaccine significantly reduced the risk of HZ [vaccine effectiveness (VE): 55%; 95% CI 52%; 58%]; VE was similar in all age groups and for individuals with chronic diseases. The HZ vaccine also reduced the risks of ophthalmic HZ (VE: 63%; 95% CI 39%; 77%) and HZ-related hospitalizations (VE: 65%; 95% CI 49%; 76%).
Another large retrospective cohort study, conducted over the same period (January 2007 to December 2009) included 766,330 immunocompetent and immunosuppressed subjects aged ≥65 years enrolled in the Medicare program [36]. VE against HZ, adjusted for age, gender, race, immunosuppression, low income, and comorbidity, was 48% (95% CI 39%; 56%) for all subjects and 51% (95% CI 41%, 59%) for immunocompetent subjects. The study reported VE against PHN at 30 and 90 days as 62% (95% CI 37%; 77%) and 59% (95% CI 21%; 79%), respectively. The third study assessed the VE of the HZ vaccine among 176,078 vaccinated and 528,234 unvaccinated KPSC enrollees. A significant risk reduction for HZ was reported for vaccinated subjects (VE: 51%; 95% CI 50%; 53%). The effectiveness was confirmed for all age groups and in patients with chronic diseases, but unlike the previous study, the VE seemed to lower in those aged 60–69 years compared with those aged ≥70 years [37].
Four smaller studies were conducted in immunocompetent populations [38–41]. The risk of recurrent HZ did not significantly differ between vaccinated and unvaccinated individuals in a matched cohort study among elderly individuals aged ≥60 years who had had a recent episode of HZ [38]. Another study reported that the overall risk of PHN was significantly reduced in immunocompetent individuals aged ≥60 years who had developed HZ, after having received the HZ vaccine (VE: 41%; 95% CI 15%; 59%) [39]. Gender-specific analysis revealed that the risk reduction for PHN was predominately observed for women with no significant difference for men. It was suggested that this gender-related difference may reflect differences in healthcare-seeking patterns but this requires further investigation. The third study reported that the VE of the HZ vaccine for HZ in immunocompetent individuals was maintained also when the vaccine was administered concomitantly with polysaccharide pneumococcal vaccine compared with non-concomitant administration, with an adjusted hazard ratio of 1.19 (95% CI 0.81; 1.74) [40]. Another matched case–control study evaluated the VE of the HZ vaccine for preventing other HZ-related outcomes, such as prodromal symptoms and medically attended prodrome, as well as HZ and PHN [41]. In Southern Minnesota, US, 266 case-patients with HZ aged ≥60 years were matched with 362 controls from January 1, 2010 to October 12, 2011. The HZ vaccine significantly prevented HZ (VE: 54%; 95% CI 32%; 69%), HZ-related prodromal symptoms (VE: 58%; 95% CI 31%; 75%) and medically attended prodrome (VE: 70%; 95% CI 33%; 87%). The HZ vaccine also significantly reduced the risk of PHN at 30 days after rash onset (VE: 61%; 95% CI 22%; 80%) [41].
HZ Vaccine Duration of Protection in Immunocompetent Adults
In clinical trials, the efficacy of HZ vaccine decreased over time. Indeed, the findings from short- and long-term persistence substudies, conducted, respectively, among 7320 vaccine and 6950 placebo recipients and 6867 vaccinees enrolled in the SPS, demonstrated that vaccine efficacy steadily declined after vaccination but remained statistically significant for HZ burden of illness (BOI) through 10 years after vaccination. On the other hand, vaccine efficacy for incidence of HZ resulted statistically significant only until 8 years after immunization [42, 43].
Recently, Tseng and colleagues confirmed the reduction of HZ vaccine efficacy in the “real-world” [37]. In the above-mentioned study performed among KPSC members aged ≥60 years vaccinated against HZ between January 1, 2007 and December 31, 2014 and 528,234 unvaccinated subjects, they evaluated the persistency of HZ VE over 8 years after immunization. The VE in reducing the incidence of HZ was reported to decline gradually over time, with figures of 68.7% (95% CI 66.3%; 70.9%) during year 1 and 49.5% (95% CI 45.7%; 53.1%) during year 2; values ranging from 39.1% (95% CI 33.8–43.9%) to 32.9% (95% CI 23.1%; 41.5%) during years 3–6; and values of 16.5% (95% CI 1.4%; 29.3%) during year 7. Eight years after immunization, VE was 4.2% (95% CI −24.0%; 25.9%) and not statistically significant.
The reduction of protection against HZ and PHN with increasing time post-vaccination has been attributed to the declining levels of vaccine-induced immunity to VZV with increasing time after vaccination, as well as to weakening host immune responses due to the aging of vaccinated population [37, 43].
HZ Vaccine Effectiveness in Immunocompromised Adults
Although the licensed HZ live-attenuated vaccine, Zostavax, has been shown to be safe and effective in older adults, it is currently not recommended for use in patients with primary and acquired immunodeficiency because of its potential to trigger the development of HZ within 4–6 weeks after vaccination [15, 30, 44–48]. However, due to the high incidence and severity of HZ and its complications in patients with immunologic disorders, vaccination could potentially offer substantial benefits for these patients. Data from “real-world” studies provide evidence that the HZ vaccine could be effective and safe in patients with immunologic disorders (Table 2) [36, 49, 50]. In one study, HZ VE was assessed in 140,923 immunocompromised individuals among whom 5531 were vaccinated [36]. The incidence rates for HZ were higher in unvaccinated immunocompromised individuals compared with vaccinated individuals and VE against HZ was estimated to be 37% (95% CI 6%; 58%), providing support for the effectiveness of HZ vaccine in these at risk individuals [36]. A retrospective cohort study assessed HZ VE and safety in a large population of 463,541 Medicare beneficiaries aged ≥60 years who had selected immune-mediated diseases, using data from 2006 to 2009 (Table 2) [49]. Vaccination was not associated with a short-term increase in the risk for varicella or HZ and was found to be associated with a decrease risk of HZ with an estimated VE of 39% (95% CI 29%; 48%). In addition, they did not identify any safety signals in vaccinated patients undergoing biologic therapy within 42 days following vaccination. These findings confirmed the results of a previous smaller retrospective study involving 44,115 younger patients with the same immune-mediated diseases enrolled in a private healthcare insurance plan from 2006 to 2009. In this study, the short-term HZ incidence rates among the vaccinated and unvaccinated individuals were similar and no safety issues were reported [51].
In another study the incidence of HZ after immunosuppressive chemotherapy was compared in 4710 individuals aged ≥60 years who had been vaccinated with HZ vaccine before and in 16,766 individuals who had not (Table 2) [50]. There were 91 (cumulative incidence 3.3%) and 583 (cumulative incidence 5.3%) HZ cases among vaccinated and unvaccinated individual, respectively, within the 30 months follow-up period, resulting in an adjusted VE of 42% (95% CI 27%; 54%). This VE in immunocompromised patients was comparable to that reported for immunocompetent individuals evaluated in their previous study, 45% (95% CI 42%; 54%), suggesting that offering HZ vaccination to patients undergoing chemotherapy in a timely manner could offer substantial benefits [35].
Real-World Safety of HZ Vaccine
Since its licensure, the safety profile of the HZ vaccine has been investigated in large studies conducted in “real-world” settings. The results have been generally consistent with those reported in clinical trials, with no safety concerns detected, in both the general population and in certain patient groups with immune-mediated conditions. In one study, using data from the Vaccine Safety Datalink Project it was reported that the HZ vaccine is safe and well-tolerated in subjects aged ≥50 years, confirming pre-licensure results [52]. In addition, in this “real-world” setting, no increased risks for cerebrovascular, cardiovascular or neurological events were observed [52]. The optimal safety profile was also established: results from a large cohort study in about 29,000 vaccinated individuals aged ≥60 years enrolled in the Kaiser Permanente Northern California reported no short-term or long-term safety issues [53].
Recently, the results from a matched case–control study of events reported in the US Vaccine Adverse Event Reporting System (VAERS) demonstrated no significantly increased risk of exacerbation or induction of severe autoimmune diseases, including Guillain–Barre syndrome, multiple sclerosis, optic neuritis, systemic lupus erythematosus, thrombocytopenia or vasculitis, after vaccination [54]. However, HZ vaccine recipients were significantly more likely to develop alopecia or arthritis than recipients of tetanus toxoid-containing vaccines.
The HZ vaccine has been reported to be safe in two studies conducted among patients undergoing immunosuppressive treatments. In particular, no adverse reactions were reported in 62 patients with hematologic malignancy following the administration of the HZ vaccine [55]. Another study, conducted using data from 14,554 adults aged ≥18 years in the Vaccine Safety Datalink Project, reported a modest increased risk of HZ within the 42 days after immunization in current immunosuppressant drug users compared with previous immunosuppressant drugs users, thus supporting the current recommendations to withhold immunosuppressant treatment for 4 weeks before HZ vaccine administration [56]. Indeed, the study authors attributed this increased risk to the reactivation of VZV latent infection rather than vaccine viral strain dissemination.
Conclusions
This comprehensive review has summarized available evidence for HZ VE and safety from studies with different designs, conducted in different populations in “real-world” settings. This evidence confirms the promising impact of the HZ vaccine on the incidence of HZ and PHN observed in the pre-licensure clinical trials in “real-world” settings. In particular, the vaccine’s good safety and tolerability profiles have been confirmed in all available studies. Robust data show that the HZ vaccine can prevent HZ and PHN in immunocompetent individuals although the VE appears to decline with increasing age and time after vaccine administration, suggesting the need for a booster dose which is currently under discussion. Additionally, a single study has provided some evidence that the HZ vaccine may also be effective for the reduction of HZ-related hospitalizations and ophthalmic HZ. Nevertheless, more studies are needed to confirm these results. Although HZ vaccine use is currently contraindicated in immunocompromised individuals, available evidence suggests there may be a potential benefit of HZ vaccine in immunosuppressed patients and in individuals with selected immune-mediated diseases in this group with a good safety profile. Thus, there is evidence that the current recommendations in these populations should be reconsidered and revised. Overall, the “real-world” data summarized here provide a reassuring picture of the HZ vaccine performance during routine clinical practice.
References
Public Health Agency of Canada. Varicella-zoster virus. Pathogen safety data sheet—infectious substances 2012. http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/var-zo-eng.php. Accessed 22 Apr 2016.
Whitley RJ. Chapter 68: Herpesviruses. In: Baron S, editor. Medical microbiology. Galveston: University of Texas Medical Branch at Galveston; 1996.
Johnson RW. Herpes zoster and postherpetic neuralgia. Expert Rev Vaccines. 2010;9(3 Suppl):21–6.
Mori I, Nishiyama Y. Herpes simplex virus and varicella-zoster virus: why do these human alphaherpesviruses behave so differently from one another? Rev Med Virol. 2005;15(6):393–406.
Cohen JI. Clinical practice: herpes zoster. N Engl J Med. 2013;369(3):255–63.
Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84(3):274–80.
Watson PN. Postherpetic neuralgia. BMJ Clin Evid. 2010;2010:519.
Berman JN, Wang M, Berry W, Neuberg DS, Guinan EC. Herpes zoster infection in the post-hematopoietic stem cell transplant pediatric population may be preceded by transaminitis: an institutional experience. Bone Marrow Transplant. 2006;37(1):73–80.
Blank LJ, Polydefkis MJ, Moore RD, Gebo KA. Herpes zoster among persons living with HIV in the current antiretroviral therapy era. J Acquir Immune Defic Syndr. 2012;61(2):203–7.
Christiansen NP, Haake RJ, Hurd DD. Early herpes zoster infection in adult patients with Hodgkin’s disease undergoing autologous bone marrow transplant. Bone Marrow Transplant. 1991;7(6):435–7.
Dunst J, Steil B, Furch S, Fach A, Bormann G, Marsch W. Herpes zoster in breast cancer patients after radiotherapy. Strahlenther Onkol. 2000;176(11):513–6.
Gnann JW, Whitley RJ. Natural history and treatment of varicella-zoster in high-risk populations. J Hosp Infect. 1991;18(Suppl A):317–29.
Gourishankar S, McDermid JC, Jhangri GS, Preiksaitis JK. Herpes zoster infection following solid organ transplantation: incidence, risk factors and outcomes in the current immunosuppressive era. Am J Transplant. 2004;4(1):108–15.
Habel LA, Ray GT, Silverberg MJ, Horberg MA, Yawn BP, Castillo AL, et al. The epidemiology of herpes zoster in patients with newly diagnosed cancer. Cancer Epidemiol Biomark Prev. 2013;22(1):82–90.
Oxman MN, Schmader KE. Editorial commentary: zoster vaccine in immunocompromised patients: Time to reconsider current recommendations. Clin Infect Dis. 2014;59(7):920–2.
Pergam SA, Forsberg CW, Boeckh MJ, Maynard C, Limaye AP, Wald A, et al. Herpes zoster incidence in a multicenter cohort of solid organ transplant recipients. Transpl Infect Dis. 2011;13(1):15–23.
Gupta G, Lautenbach E, Lewis JD. Incidence and risk factors for herpes zoster among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4(12):1483–90.
Marehbian J, Arrighi HM, Hass S, Tian H, Sandborn WJ. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2009;104(10):2524–33.
Nagasawa K, Yamauchi Y, Tada Y, Kusaba T, Niho Y, Yoshikawa H. High incidence of herpes zoster in patients with systemic lupus erythematosus: an immunological analysis. Ann Rheum Dis. 1990;49(8):630–3.
Ranganathan P. Herpes zoster infection risk in patients with rheumatoid arthritis on tumour necrosis factor inhibitors. Ann Rheum Dis. 2013;72(5):e4.
Smitten AL, Choi HK, Hochberg MC, Suissa S, Simon TA, Testa MA, et al. The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum. 2007;57(8):1431–8.
Strangfeld A, Listing J, Herzer P, Liebhaber A, Rockwitz K, Richter C, et al. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-tnf-alpha agents. JAMA. 2009;301(7):737–44.
Veetil BM, Myasoedova E, Matteson EL, Gabriel SE, Green AB, Crowson CS. Incidence and time trends of herpes zoster in rheumatoid arthritis: a population-based cohort study. Arthritis Care Res (Hoboken). 2013;65(6):854–61.
Wolfe F, Michaud K, Chakravarty EF. Rates and predictors of herpes zoster in patients with rheumatoid arthritis and non-inflammatory musculoskeletal disorders. Rheumatology (Oxford). 2006;45(11):1370–5.
Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833.
Drolet M, Brisson M, Schmader KE, Levin MJ, Johnson R, Oxman MN, et al. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: a prospective study. CMAJ. 2010;182(16):1731–6.
Johnson RW, Bouhassira D, Kassianos G, Leplege A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med. 2010;8:37.
Food and Drug Administration. News and events 2006. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108659.htm. Accessed 22 Apr 2016.
European Medicines Agency. Zostavax 2016. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000674/human_med_001185.jsp&mid=WC0b01ac058001d124. Accessed 22 Apr 2016.
Centers for Disease Control and Prevention. Update on herpes zoster vaccine: licensure for persons aged 50 through 59 years. MMWR. 2011;60(44):1528.
Centers for Disease Control and Prevention. Shingles vaccination: what everyone should know 2016. http://www.cdc.gov/vaccines/vpd-vac/shingles/vacc-need-know.htm. Accessed 22 Apr 2016.
Centers for Disease Control and Prevention. Update on recommendations for use of herpes zoster vaccine. MMWR. 2014;63(33):729–31.
Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–84.
Schmader KE, Levin MJ, Gnann JW Jr, McNeil SA, Vesikari T, Betts RF, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis. 2012;54(7):922–8.
Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011;305(2):160–6.
Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med. 2013;10(4):e1001420.
Tseng HF, Harpaz R, Luo Y, Hales CM, Sy LS, Tartof SY, et al. Declining effectiveness of herpes zoster vaccine in adults aged ≥60 years. J Infect Dis. 2016;213(12):1872–5.
Tseng HF, Chi M, Smith N, Marcy SM, Sy LS, Jacobsen SJ. Herpes zoster vaccine and the incidence of recurrent herpes zoster in an immunocompetent elderly population. J Infect Dis. 2012;206(2):190–6.
Tseng HF, Lewin B, Hales CM, Sy LS, Harpaz R, Bialek S, et al. Zoster vaccine and the risk of postherpetic neuralgia in patients who developed herpes zoster despite having received the zoster vaccine. J Infect Dis. 2015;212(8):1222–31.
Tseng HF, Smith N, Sy LS, Jacobsen SJ. Evaluation of the incidence of herpes zoster after concomitant administration of zoster vaccine and polysaccharide pneumococcal vaccine. Vaccine. 2011;29(20):3628–32.
Marin M, Yawn BP, Hales CM, Wollan PC, Bialek SR, Zhang J, et al. Herpes zoster vaccine effectiveness and manifestations of herpes zoster and associated pain by vaccination status. Hum Vaccin Immunother. 2015;11(5):1157–64.
Schmader KE, Oxman MN, Levin MJ, Johnson G, Zhang JH, Betts R, Morrison VA, Gelb L, Guatelli JC, Harbecke R, Pachucki C, Keay S, Menzies B, Griffin MR, Kauffman C, Marques A, Toney J, Keller PM, Li X, Chan IS, Annunziato P, Shingles Prevention Study Group. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis. 2012;55(10):1320–8.
Morrison VA, Johnson GR, Schmader KE, Levin MJ, Zhang JH, Looney DJ, Betts R, Gelb L, Guatelli JC, Harbecke R, Pachucki C, Keay S, Menzies B, Griffin MR, Kauffman CA, Marques A, Toney J, Boardman K, Su SC, Li X, Chan IS, Parrino J, Annunziato P, Oxman MN, Shingles Prevention Study Group. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis. 2015;60(6):900–9.
Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the advisory committee on immunization practices (acip). MMWR. 2008;57(Rr-5):1–30.
Maggi S, Gabutti G, Franco E, Bonanni P, Conversano M, Ferro A, et al. Preventing and managing herpes zoster: key actions to foster healthy aging. Aging Clin Exp Res. 2015;27(1):5–11.
Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–238.
Sartori AM. A review of the varicella vaccine in immunocompromised individuals. Int J Infect Dis. 2004;8(5):259–70.
Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American college of rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762–84.
Zhang J, Xie F, Delzell E, Chen L, Winthrop KL, Lewis JD, et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA. 2012;308(1):43–9.
Tseng HF, Tartof S, Harpaz R, Luo Y, Sy LS, Hetcher RC, et al. Vaccination against zoster remains effective in older adults who later undergo chemotherapy. Clin Infect Dis. 2014;59(7):913–9.
Zhang J, Delzell E, Xie F, Baddley JW, Spettell C, McMahan RM, et al. The use, safety, and effectiveness of herpes zoster vaccination in individuals with inflammatory and autoimmune diseases: a longitudinal observational study. Arthritis Res Ther. 2011;13(5):R174.
Tseng HF, Liu A, Sy L, Marcy SM, Fireman B, Weintraub E, et al. Safety of zoster vaccine in adults from a large managed-care cohort: a Vaccine Safety Datalink study. J Intern Med. 2012;271(5):510–20.
Baxter R, Tran TN, Hansen J, Emery M, Fireman B, Bartlett J, et al. Safety of Zostavax—a cohort study in a managed care organization. Vaccine. 2012;30(47):6636–41.
Lai YC, Yew YW. Severe autoimmune adverse events post herpes zoster vaccine: a case-control study of adverse events in a national database. J Drugs Dermatol. 2015;14(7):681–4.
Naidus E, Damon L, Schwartz BS, Breed C, Liu C. Experience with use of Zostavax(®) in patients with hematologic malignancy and hematopoietic cell transplant recipients. Am J Hematol. 2012;87(1):123–5.
Cheetham TC, Marcy SM, Tseng HF, Sy LS, Liu IL, Bixler F, et al. Risk of herpes zoster and disseminated varicella zoster in patients taking immunosuppressant drugs at the time of zoster vaccination. Mayo Clin Proc. 2015;90(7):865–73.
Acknowledgments
No funding was received for this work. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. Editorial assistance for the preparation of this manuscript was provided by Dr Margaret Haugh of MediCom Consult, funded by Sanofi Pasteur MSD. Funding for the article processing charges and open access fee was provided by Sanofi Pasteur MSD.
Disclosures
Filippo Ansaldi and Giancarlo Icardi have previously participated at speaker’s bureaus and advisory board meetings sponsored by GSK, Pfizer, Novartis and Sanofi Pasteur and have received research funding as investigator or principal investigator from GSK, Pfizer, Novartis and Sanofi Pasteur MSD. Cecilia Trucchi, Cristiano Alicino, Chiara Paganino and Andrea Orsi declare that they have no conflict of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to www.medengine.com/Redeem/36D4F060739D7E0A.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ansaldi, F., Trucchi, C., Alicino, C. et al. Real-World Effectiveness and Safety of a Live-Attenuated Herpes Zoster Vaccine: A Comprehensive Review. Adv Ther 33, 1094–1104 (2016). https://doi.org/10.1007/s12325-016-0355-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-016-0355-0