Abstract
Introduction
This study compared the efficacy and safety of two mesalazine formulations in the treatment of Chinese patients with mildly to moderately active ulcerative colitis (UC).
Methods
In this multicenter, single-blind, randomized controlled study of 251 patients with active UC conducted from November 2010 to January 2012, subjects were randomized to treatment with mesalazine modified-release tablets (MR group, n = 123) or enteric-coated tablets (EC group, n = 128) at 800 mg three-times daily for 8 weeks. The primary efficacy measure was the decrease in UC Disease Activity Index (UCDAI) at final evaluation. If the 95% confidence interval (CI) lower limit of the difference of the decrease in UCDAI between groups was over −1.0, mesalazine modified-release tablets were considered non-inferior to mesalazine enteric-coated tablets. The change in UCDAI in patients with mild and moderate (UCDAI 3–5 and 6–8 at enrollment, respectively) UC was analyzed. Secondary efficacy measures were remission and efficacy rates. Incidences of adverse drug reactions (ADRs) were calculated.
Results
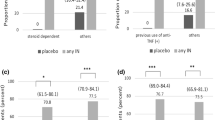
The decreases in UCDAI at final evaluation were 2.84 and 2.56 in the MR and EC groups, respectively, with a difference of 0.27 between groups (95% CI −0.34, 0.88). The remission rates were 48.33% (58/120) and 55.65% (69/124), and the efficacy rates were 63.33% (76/120) and 66.94% (83/124) in the MR and EC groups, respectively (all P > 0.05). In patients with mild UC, the decreases in UCDAI were 2.16 and 2.05 in the MR and EC groups, respectively, while in patients with moderate UC they were 3.49 and 3.03, respectively (all P > 0.05). The incidences of ADRs in the MR and EC groups were 6.61% (8/121) and 10.24% (13/127), respectively (P > 0.05). No serious ADRs were reported during the study.
Conclusion
Mesalazine modified-release tablets are non-inferior to enteric-coated tablets and are an effective and safe treatment option in Chinese patients with mildly to moderately active UC.
Trial registration
ClinicalTrials.gov identifier: NCT01257386.
Funding
Tillotts Pharma AG.

Similar content being viewed by others
References
Chinese Medical Association. Chinese consensus on standard management of inflammatory bowel diseases. Zhonghua Xiao Hua Za Zhi. 2007;27:545–50.
Kornbluth A, Sachar DB. Ulcerative colitis practice guideline in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2004;99:1371–85.
Brogden RN, Sorkin EM. Mesalazine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in chronic inflammatory bowel disease. Drugs. 1989;38:500–23.
Myers B, Evans DN, Rhodes J, et al. Metabolism and urinary excretion of 5-amino salicylic acid in healthy volunteers when given intravenously or released for absorption at different sites in the gastrointestinal tract. Gut. 1987;28:196–200.
Mardini HA, Lindsay DC, Deighton CM, Record CO. Effect of polymer coating on faecal recovery of ingested 5-amino salicylic acid in patients with ulcerative colitis. Gut. 1987;28:1084–9.
Oliveira L, Cohen RD. Maintaining remission in ulcerative colitis—role of once daily extended-release mesalamine. Drug Des Devel Ther. 2011;5:111–6.
Riley SA, Tavares IA, Bennett A, Mani V. Delayed-release mesalazine (5-aminosalicylic acid): coat dissolution and excretion in ileostomy subjects. Br J Clin Pharmacol. 1988;26:173–7.
Sutherland LR, Martin F, Greer S, et al. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology. 1987;92:1894–8.
Ito H, Iida M, Matsumoto T, et al. Direct comparison of two different mesalamine formulations for the induction of remission in patients with ulcerative colitis: a double-blind, randomized study. Inflamm Bowel Dis. 2010;16:1567–74.
Gisbert JP, Gomollon F, Mate J, Pajares JM. Role of 5-aminosalicylic acid in treatment of inflammatory bowel disease: a systematic review. Dig Dis Sci. 2002;47:471–88.
Sninsky CA, Cort DH, Shanahan F, et al. Oral mesalamine (Asacol) for mildly to moderately active ulcerative colitis. A multicenter study. Ann Intern Med. 1991;115:350–5.
Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–9.
Hiwatashi N, Suzuki Y, Mitsuyama K, Munakata A, Hibi T. Clinical trial: effects of an oral preparation of mesalazine at 4 g/day on moderately active ulcerative colitis. A phase III parallel-dosing study. J Gastroenterol. 2011;46:46–56.
Lichtenstein GR, Kamm MA, Boddu P, et al. Effect of once- or twice-daily MMX mesalamine (SPD476) for the induction of remission of mild to moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2007;5:95–102.
Kamm MA, Sandborn WJ, Gassull M, et al. Once-daily, high-concentration MMX mesalamine in active ulcerative colitis. Gastroenterology. 2007;132:66–75.
Prantera C, Kohn A, Campieri M, et al. Clinical trial: ulcerative colitis maintenance treatment with 5-ASA: a 1-year, randomized multicentre study comparing MMX with Asacol. Aliment Pharmacol Ther. 2009;30:908–18.
Jing Sun, Yaozong Yuan. Mesalazine modified-release tablet in the treatment of ulcerative colitis in active phase: a multi-center, single-blinded and randomized controlled study. Chin J Digest. 2015;35:252–5.
Acknowledgments
The general contents of this manuscript have been published in the Chinese Journal of Digestion 2015;35(4):252–5 [17], and are included here with permission. The funding for this study was provided by Tillotts Pharma AG. The article processing charges for this publication were funded by Tillotts Pharma AG. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. We thank Yuichi Takano (Zeria Pharmaceutical Co., Ltd., Tokyo, Japan) for providing assistance in writing this paper. Editorial assistance in the preparation of this manuscript was provided by Dr. Michelle Belanger on behalf of Springer Healthcare Communications. Support for this assistance was funded by Tillotts Pharma AG. We would like to thank the following doctors and hospitals for their contributions to this study: Prof. Ran Zhihua, Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine; Prof. Li Zhaoshen, Changhai Hospital Affiliated to Second Military Medical University; Prof. Lu Lungen, Shanghai First People Hospital; Prof. Shen Xizhong, Zhongshan Hospital, Fudan University; Prof. Zhang Zhenyu, Nanjing First Hospital; Prof. Shi Ruihua, the First Hospital, Nanjing Medical University; Prof. Zou Xiaoping, Gulou Hospital, Nanjing University School of Medicine; Prof. Hou Xiaohua, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology; Prof. Luo Hesheng, Renmin Hospital of Wuhan University; Prof. Li Youming, The First Affiliated Hospital of College of Medicine, Zhejiang University; Prof. Cai Jianting, The Second Affiliated Hospital of College of Medicine, Zhejiang University; Prof. Xie Pengyan, Peking University First Hospital; Prof. Zhang Shutian, Prof. Wu Yongdong, Beijing Friendship Hospital, Capital Medical University; Prof. Hao Jianyu, Beijing Chao-Yang Hospital, Capital Medical University; Prof. Wang Bangmao, Tianjin Medical University General Hospital; Prof. Sheng Jianqiu, The Military General Hospital of Beijing PLA; and Prof. Li Liangping, Sichuan Provincial People’s Hospital.
Disclosures
Jing Sun has received honoraria from Tillotts Pharma AG. Yaozong Yuan has received honoraria from Tillotts Pharma AG. The authors declare no conflict of interest directly associated with the content of this manuscript.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content for this article can be found at http://www.medengine.com/Redeem/6644F06003969091.
Rights and permissions
About this article
Cite this article
Sun, J., Yuan, Y. Mesalazine Modified-Release Tablet in the Treatment of Ulcerative Colitis in the Active Phase: A Chinese, Multicenter, Single-Blind, Randomized Controlled Study. Adv Ther 33, 400–409 (2016). https://doi.org/10.1007/s12325-016-0303-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-016-0303-z