Abstract
Pain presents in 80% of patients with advanced cancer, and 30% have periods of increased pain due to fluctuating intensity, known as breakthrough cancer pain (BTcP). BTcP is high-intensity, short-duration pain occurring in several episodes per day and is non-responsive to treatment. The clinical approach to BTcP is variable. A review of the literature was performed to provide clinicians and practitioners with a rational synthesis of the ongoing scientific debate on BTcP and to provide a basis for optimal clinical approach to BTcP in adult Italian patients. Data show that circadian exacerbations of pain should be carefully monitored, differentiating, if possible, between fluctuations of background pain (BP), end-of-dose effect, and BTcP. BTcP should be monitored in all care contexts in clinical practice and each care facility must have all the medications and products approved for use in BTcP at their disposal. Data show that knowledge about medications for BTcP is lacking: medications for BTcP treatment are not interchangeable, although containing the same active substance; each physician must know the specific characteristics of each medication, its pharmacological properties, limitations in clinical practice, specifics relating to titration and repeatability of administration, and technical specifics relating to the accessibility and delivery. Importantly, before choosing a rapid-onset opioid (ROO), it is essential to deeply understand the status of patient and the characteristics of their family unit/caregivers, taking into account the patient’s progressive loss of autonomy and/or cognitive-relational functionality. When BTcP therapy is initiated or changed, special attention must be paid to training the patient and family members/caregivers, providing clear instructions regarding the timing of drug administration. The patient must already be treated effectively with opioids before introducing ROOs for control of BTcP.
Similar content being viewed by others
Introduction
It is commonly experienced by clinicians involved in cancer treatment, especially for patients in the advanced and progressive phase of disease, that pain is not always adequately controlled, even when up-to-date treatment guidelines are followed. One of the most frequent causes of such difficulty relates to the observation that pain occurs in 80% of cancer patients in an advanced stage of disease and in 30% of cases with a high intensity of pain [1]. These pain fluctuations are often unexpected and unpredictable [1]. Sometimes, they can be due to predictable, although unavoidable, causes such as voluntary motor activity or automatic changes in sleeping position [2].
In the last 20 years, the objective analysis of the clinical pathway in oncologic patients has allowed to identify, within these pain variations, a specific pain syndrome called breakthrough cancer pain (BTcP) by the international scientific community, also defined as intense episodic pain (dolore episodico intenso) by Italian physicians.
BTcP is differentiated from background pain (BP) variations by: (a) its high intensity, generally ≥7 in a Numerical Rating Scale (NRS) 0–10; (b) a short time between onset and peak of intensity (a few minutes); (c) a short duration (approximately 60 min); (d) its potential recurrence during 24 h (3–4 daily episodes in most patients); and (e) non-responsiveness to treatment for BP, even when the daily dose of medication (primarily opioids) is increased [3]. Even today, the clinical approach to BTcP varies markedly among physicians, from a complete negation of the syndrome to its over-estimation.
The primary objective of this paper was to provide clinicians and practitioners involved in treatment of cancer patients in different roles with a reasoned synthesis of the ongoing scientific debate on BTcP. The debate is dynamic, as inferred from the considerable body of literature annually produced at both international and national level, and from the numerous scientific meetings during congresses or single-topic meetings held each year. Our analysis aims at providing the basis for an optimal clinical approach to BTcP.
Methods
This paper is the result of a debate among three Italian experts—two clinicians and a pharmacologist—operating in pain therapy and palliative care. The integration of an analysis of existing literature and clinical experience of the authors offers a rational and up-to-date support to all who are asked to provide an adequate treatment for pain to over 180,000 oncologic patients in Italy. Some of these are terminally ill cancer patients experiencing the so-called advanced and progressive stage of disease, no longer manageable with etiologic treatments. This article does not contain any new studies with human or animal subjects performed by any of the authors.
Pathophysiology of BTcP
Definitions of BTcP
Despite increasing availability of ever more accurate tools and studies, the clinical features and physiopathogenesis of BTcP remain unclear. Over time, numerous differing BTcP definitions have been reported in the literature. All of them, however, derive from the first definition of the clinical profile of BTcP described as “a transitory flare of pain in the setting of chronic pain managed with opioid therapy” [4]. In 1995, BTcP was described as “an exacerbation of pain that occurs spontaneously or which accompanies a specific activity” [5]. More recently, BTcP has been defined as “a pain of short duration, more or less intense, which breaks through the pain barrier provided by analgesic medications managing BP” [6, 7].
According to one of the more recent and comprehensive definitions, “breakthrough pain is a transient exacerbation of pain that occurs either spontaneously, or in relation to a specific predictable or unpredictable trigger, despite relatively stable and adequately controlled background pain” [e.g., background pain controlled through an around-the-clock (ATC) dosing, that is drug administration at fixed times] [2]. A year earlier, the idea of BTcP having a different causal mechanism than for BP was introduced: “Breakthrough pain can be an exacerbation of the baseline pain OR it can be a pain with a different cause from that of the baseline pain” [1]. According to pathogenetic interpretation, BTcP should no longer be considered a fluctuation or a sudden variation of BP, but a type of pain triggered by a different causal mechanism, superimposed on the pre-existing mechanism causing BP.
The definition by Davies et al. [2] has been revised in recent publications [3, 8]. An extensive survey of clinicians working in selected Italian centers for palliative care and pain therapy led to reformulate recommendations for the best practice in BTcP diagnosis and treatment. The collected opinions and suggestions resulted in a more complex and analytic BTcP definition: “BTcP is an exacerbation of pain of high intensity, with a difference of at least 3 points compared to background pain and with an absolute intensity ≥7 points (measured with a NRS), with a daily frequency typically not exceeding 4 episodes, which occur either spontaneously or as a result of predictable or unpredictable triggering factors, despite an adequately controlled background pain (average pain in the last 24 h, ≤4 points) with an around the clock opioid therapy” [3]. Although deriving from an exclusively Italian expert panel and not yet validated by an international consensus, this definition is useful for enabling clinicians to understand BTcP characteristics and to better guide them in recognizing its presence. The definition specifically describes the phenomenon of BTcP and highlights both clinical and pathogenetic aspects.
Thus, according to previously reported considerations, BTcP cannot be recognized as a single nosological entity, but includes different and changing pathogenetic mechanisms justifying its sub-typing [3].
BTcP: Clinical Characteristics and Prevalence
The poorly defined and variable clinical characteristics of BTcP, together with imprecise prevalence data, account for the discordant taxonomy for this type of pain. Data show that BTcP is present in 40–80% of patients, characterized by a rapid onset (<3 min), a short duration (median 30 min), and a daily frequency of 4–7 episodes/day [9–11]. The prevalence between 40% and 80% is inaccurate. Furthermore, the definitions “rapid onset” and “short duration” are both qualitative and the daily average frequency, between 4 and 7 episodes, is too wide.
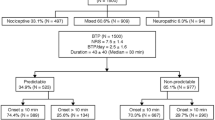
For clinical purposes, representation of BTcP types is shown in Fig. 1: BTcP is usually classified into a stimulus-independent or spontaneous BTcP, a stimulus-dependent or evoked BTcP and a BTcP related to the therapeutic approach or to procedural interventions [12]. Evoked BTcP may be volitional or non-volitional. Evoked volitional BTcP may be related to normally painful stimuli (e.g., pinprick, application of intense heat) or to stimuli that do not normally provoke pain (allodynia). The attribute “non-volitional” refers to mechanisms regulated by the autonomous nervous system, such as intestinal peristalsis, arterial pulsation, and body temperature.
As shown in Fig. 1, one pain type originally traced to BTcP is closely related to the loss of antalgic efficacy caused by the end-of-dose effect; according to available data, this type of pain would account for 17–30% of episodes at first classified as BTcP [13]. In these patients, a pain exacerbation would be brought on by an inadequate treatment of BP, in terms of dose/duration of efficacy/schedule of administration of medications used according to the ATC plan, at fixed times daily.
Zeppetella [13], and most other authors, do not include end-of-dose pain episodes among BTcP, as a fundamental requirement of BTcP is that BP must be properly controlled. This type of pain is due to inadequate BP therapy and should be treated in different ways.
Conversely, most authors include the incident pain in BTcP, characterized by a prevalence of 50–60%. It is produced by a causal stimulus superimposing on a basal pathological condition and can be distinguished in: (a) predictable incident pain caused by a gentle pressure, a movement, cough, swallowing, chewing, etc., which can be predicted and adequately pre-treated; and (b) unpredictable incident pain, for example associated with intestinal peristalsis, a spastic contraction of the hollow viscus (e.g., pain of colic type), an ischemic event, etc. [2].
Lastly, idiopathic or spontaneous pain (20–50% prevalence) is a type of BTcP not associated with a specific, recognizable pathogenesis. The concept of “spontaneous” is related to a total lowering of the receptor threshold so that the pain is present even in the absence of external stimuli [13].
According to recommendations provided by the task force of the Association for Palliative Medicine of Great Britain and Ireland, a close relationship exists between BTcP definition and physiopathological classification [2]: BTcP is deemed a range of entities, the physiopathogenetic mechanisms involved are multiple (nociceptive, neuropathic, and mixed), and idiopathic or spontaneous BTcP should be differentiated from incident BTcP, with the latter further divided into subcategories (Table 1) [2, 8].
The difficulty, not fully solved in clinical practice, is due to the incapability to distinguish a BP variation from an actual BTcP episode. Thus, therapeutic behaviors differ substantially; for example, some authors inappropriately treat all circadian flares of pain with rescue opioids, as BP fluctuations [8].
BTcP: Pathogenesis
Pathogenesis underlying BTcP onset is probably heterogeneous. BTcP may depend on stimuli responsible for a sudden excess of afferent nerve impulses or on alterations originating in the somatosensory system [14]. BTcP pathogenetic hypotheses are shown in Table 2.
A first possible mechanism involved in BTcP onset could be related to a transient increase in afferent stimuli secondary to nearby tissue involvement following primary (or secondary) neoplastic lesion-dependent factors, or following the occurrence of additional algogenic stimuli originating from neoplastic tissue. The neoplastic mass may cause a transient stimulation through involvement of nearby sensitive structures (e.g., nerve compression). At the same time, a new stimulus (e.g., secondary to a movement or swallowing), not necessarily painful in normal conditions, can exceed the nociceptor threshold and produce an intense pain (mechanism: allodynia from non-painful stimulus and hyperalgesia from painful stimulus). One of the clinical characteristics of this type of pain is that it can be confined to the originating tissue or, when the neoplasm affects adjacent tissues, the pain also occurs in other sites (e.g., innervation area of nerve structures involved). Pain duration is generally linked to the stimulus duration (e.g., movement, posture or changes of posture, defecation, etc.) [15].
A second possible mechanism involved in the occurrence of BTcP is the increase in peripheral sensitization of tissue terminals, of nociceptors or ectopic sites formed following cancer-induced anatomical-functional changes that lower the nociceptor or ectopic site threshold [15].
A third mechanism for BTcP onset is an increase in spinal neuron sensitization—the central sensitization—following the spatial–temporal increase of afferents originating from peripheral receptors, not activated in normal conditions. This can happen in cases where sensitive input delivered by the C fibers increases, following the engagement of the so-called “silent” nociceptors [16]. Silent nociceptors are located in the visceral system in large numbers, for example in the intestine, and so are not activated under physiological conditions (e.g., in the absence of chronic inflammation).
The Italian Breakthrough/Episodic Pain Study Group provided important data on various BTcP effects in different body regions, indirectly confirming the third pathogenetic mechanism: BTcP would occur more frequently in the gastrointestinal and urogenital tracts, in the breast and in the lung, because these are anatomical structures richer in silent receptors and Aδ fibers, activated by mechanical and chemical stimuli linked to the presence of neoplastic tissue [17].
If pain is due to the third mechanism, precisely because of the functional characteristics of the spinal neurons which have been sensitized, the perception of pain extends into broader areas than BP and persists for a longer time; it differs from what occurs when the first two “peripheral” mechanisms are involved [18].
According to this analysis of BTcP pathogenesis, when BTcP is located in the BP area, it is probably due to peripheral mechanisms. If sudden acute exacerbation is perceived in a more extensive area than that of BP (without following any particular innervation or organ area), probably the spinal and supraspinal neuronal systems are already predominantly involved [18].
Lastly, the occurrence of a sudden pain flare, even with BTcP characteristics, in an area different from the main neoplasm may be secondary to a metastatic localization.
Pharmacological Aspects and BTcP
Among the administration routes used for fentanyl—the active substance mainly used for treating BTcP—the transmucosal routes (buccal, sublingual, or nasal) are the most common.
Characteristics differentiating the transmucosal routes used to administer fentanyl and the preparations currently available in Italy are analyzed below.
Oral Mucosa: General Characteristics and Transmucosal Routes of Administration
The oral mucosa is provided with physiological properties that are well suited to pharmacological administration, by virtue of its wide surface, uniform temperature, high vascularization, and permeability. It therefore offers favorable conditions for rapid absorption, representing an ideal administration route particularly suitable for pathological states that require a rapid therapeutic response, such as BTcP. Oral transmucosal administration also excludes the liver filter, eliminating the first-pass effect and accelerating therapeutic action [19].
Importantly, epithelial cells forming oral mucosa are not in contact with each other through tight junctions (typical intestinal and nasal mucosa junctions) but through desmosomes and hemidesmosomes, loose intercellular junctions which make the transport and flux of substances easier [19].
In the oral cavity, we can find separate areas pertaining to the palatal mucosa, the gingival mucosa, the so-called buccal mucosa pertaining to the cheeks and the sublingual mucosa. Sublingual and buccal mucosae, not keratinized, better work for the absorption of substances; however, the greater thickness of the buccal mucosa, corresponding to 500–600 μm, reduces its permeability [19]. The reduced thickness and the high degree of permeability of the sublingual mucosa, compared to the buccal mucosa, make this area the most favorable for absorption of substances [19].
A more thorough exploration of the multilayered epithelium lining the oral mucosa allows to distinguish the layer formed by the so-called prickle cells or spinous cells from which granules of phospholipid material are interposed and disseminated among the epithelial cells (Fig. 2). The phospholipidic composition of this substance, although partly a barrier, helps to create a mobile intercellular space allowing the flow of substances [19].
In short, two transit routes through the oral mucosa may be recognized for substances as well as for medications: the transcellular route, a pathway for liposoluble substances (such as fentanyl), able to pass through the cell membranes; and the paracellular route, preferred by more water-soluble substances, which flow through the intercellular phospholipid material (Fig. 3) [19].
The number of medications administered orally that can take advantage of the transcellular route is limited because these substances must have certain physical and chemical properties dominated by a precise balance between water solubility and lipophilicity [20].
An additional element implied in substance absorption through the oral mucosa is represented by saliva, which has multiple physiological functions. Salivary glands collectively produce more than 1 L of saliva per day. They are classified into major and minor salivary glands. The former are mainly responsible for the aqueous component of saliva, whereas the latter, and particularly the sublingual glands, are responsible for the viscous component of saliva, which is enriched in mucins [20].
The Sublingual Administration Route
A study carried out in 1998 reported that, although relatively more permeable than the buccal mucosa, the sublingual mucosa does not provide a suitable transmucosal administration route: the sublingual region is devoid of an underlying muscular reinforcement and support structure, which is present in the buccal mucosa and confers fixedness and firmness to the epithelium [20]. Furthermore, the sublingual epithelium is constantly washed by huge amounts of saliva that makes the persistence of the drugs under the tongue difficult. Thus, in accordance with this study, the sublingual mucosa, while ensuring a rapid onset of pharmacological action because of its high permeability and abundant blood supply, would offer an effective route of administration only for quickly absorbed medications [21]. However, drug delivery can also be affected by the concentration of mucus in the saliva; when the medication adheres to mucus it does not undergo easy removal by saliva, but its contact with mucosa epithelium lasts longer and its absorption continues with more efficacy. Mucoadhesive substances have been formulated to block the medication at the sublingual level [22].
A recent study showed that transmucosal administration efficiency is limited by factors that support the presence of free, and therefore ready to be swallowed, medication in the oral cavity [23]. Among these factors, salivary secretion in its aqueous component plays a major role as it causes the release of medication in the oral cavity. However, if the medication adheres to the mucosa, absorption is guaranteed and systemic exposure will be largely determined by the physical and chemical properties of the medication [23].
It has been reported that buccal and sublingual fat may absorb buprenorphine, thus delaying its plasma level increase and half-life [24]. Since fentanyl shares similar lipophilic properties, it is possible that buccal fat retention occurs. However, to the best of our knowledge, this does not seem to be a major determinant in the absorption of oral transmucosal fentanyl.
Pharmacological Properties of Fentanyl
Pharmacodynamics
Fentanyl, a full µ-opioid receptor agonist, is a synthetic opioid with rapid onset of action and short duration (indicated for BTcP) and with a potency 50–100 times greater than that of morphine. In oral transmucosal formulations, its analgesic efficacy, proportional to the plasma concentration, occurs at between 0.3 and 1.2 ng/mL of blood, while respiratory depression is observed at between 10 and 20 ng/mL [25].
Pharmacokinetics
Fentanyl is a highly lipophilic molecule, capable of rapidly crossing the blood–brain barrier, which undergoes a rapid sublingual absorption, and is metabolized by CYP3A4, a cytochrome largely responsible for pharmacological interactions; therefore, particular attention must be paid to concomitant medications [25]. Furthermore, it should be taken in consideration that fentanyl kinetics may be affected over time by accumulation in fat and muscle, that, when saturated after chronic and repeated dosing, may cause a prolongation of fentanyl half-life, that, in turn, might be life-threatening.
Fentanyl is available in Italy in the following formulations:
-
oral transmucosal fentanyl citrate (ACTIQ®, TEVA, Milan, Italy);
-
fentanyl buccal tablets (EFFENTORA®, Cephalon Europe, now TEVA, Haarlem, The Netherlands);
-
fentanyl sublingual tablets (ABSTRAL®, ProStrakan, Galashiels, UK);
-
fentanyl intranasal spray (INSTANYL®, Takeda Pharmaceuticals, Zurich, Switzerland); and
-
fentanyl intranasal spray with pectin (PecFent®, Archimedes Pharma, Reading UK).
In Italy, since 2014, the fentanyl BioErodible MucoAdhesive (BEMA) disk (BREAKYL®, Meda Pharma GmbH & Co. KG, Bad Homburg, Germany) is available, while fentanyl sublingual spray is not yet available. Each administration route offers benefits and risks, as shown in Table 3 [25–32].
Buccal and Sublingual Formulations: Data From the Literature
A recently published review compared the pharmacokinetic profile of two different transmucosal formulations and an intranasal formulation of fentanyl (Actiq, Effentora, and Instanyl, respectively), emphasizing that the formulation should be selected according to patient needs, the evolution of pain, and to its onset and persistence [33].
A study carried out in 2006 assessed the bioequivalence of equal doses of fentanyl via the buccal route when administered in four tablets of 100 μg or in a single tablet of 400 μg [34]. The study showed that 400 μg of Effentora in a single tablet and four Effentora tablets each of 100 μg, administered via the buccal route are not bioequivalent by virtue of the different absorption surfaces exposed [34]. In spite of this evidence, a study carried out in 2008 demonstrated the bioequivalence between buccal and sublingual use of Effentora in 400 μg tablets in healthy volunteers [35]. This is in contrast with the idea that if a medication with no mucoadhesive molecules is placed under the tongue, its absorption should be reduced versus buccal administration (Fig. 4a, b) [35]. Furthermore, the findings of this study could be considered valid only for the dosage of 400 µg and could not be extrapolated to other dosages; the bioequivalence refers only to 400 μg and the bioequivalence of other dosages would require confirmation in a clinical trial.
Fentanyl plasmatic concentrations and pharmacokinetic parameters related to buccal and sublingual routes. a Logarithm of the plasmatic concentrations of fentanyl after administration of a single dose of 400 μg in tablet via the buccal and sublingual routes and 4. b The relative pharmacokinetic parameters [35]. FBT fentanyl buccal tablet. Reproduced with permission from Darwish et al. [35]
In agreement with this statement, a recent paper published in the New England Journal of Medicine by members of the US Food and Drug Administration shows that budeprion, the generic version of bupropion, was bioequivalent to the branded drug at the dosage of 150 mg, but not at the dosage of 300 mg, in fact suggesting that bioequivalence at different dosages should be demonstrated by clinical studies and not extrapolated [36].
There are substantial differences between medications formulated for sublingual administration, such as Abstral, and buccal administration, such as Effentora. Unlike Abstral, Effentora excipients do not include the mucoadhesives that give Abstral sublingual absorption capability; specifically, crosscarmellose, a powerful disintegrant improver of absorption with bio-mucoadhesive action, and the silicified microcrystalline cellulose, a tablet binder and, concurrently, an agent promoting disintegration and bio-mucoadhesion [37].
Diversification of Therapeutic Approaches in BTcP
In 2009, specific recommendations for the management of BTcP including an algorithm for dose titration were published [2]. On lack of pain control or in the presence of adverse effects with oral transmucosally administered opioids, the medication dose should be titrated. Titration is essential, because the characteristics of the oral mucosa are different among patients and BTcP management needs to be personalized; this implies that adequate therapeutic tools must be available. The currently available tools are multiple and varied, but the superiority of one product compared to another cannot be stated, rather only the validity and efficacy of one product in relation to the needs, individual characteristics, and pain of an individual patient. Therefore, it is not correct to consider different formulations as equivalent: sublingual administrations must be recognized as such, and distinguished from other types of transmucosal administration. Maintaining a diversification of therapeutic approaches, on the basis of patient’s biological complexity and the pharmacological differences of each transmucosal formulation, allows the patient to be offered an extensive range of therapeutic equipment from which to draw for individual needs.
The Intranasal Route
The intranasal route is another important non-invasive route for systemic administration and, like the oral transmucosal route, offers benefits of rapid absorption, absence of first-pass metabolism, and a rapid therapeutic response. The respiratory area around the inferior turbinate is the area of maximum absorption of medications due to its extended surface, high permeability, and abundant vascularization [38]. The epithelium lining the nasal cavity consists of basal cells, ciliated cells and mucus-secreting cells (“goblet cells”). Unlike the oral mucosa, the intercellular junctions are tight, restricting the passage of substances [38]. Transcellular and paracellular passage can be recognized.
The currently available spray formulation, Instanyl, in which the medication is passively absorbed, presents a limitation due to the variable amount of solution which enters the pharynx and then is swallowed. In an attempt to overcome this problem, a new formulation of fentanyl was devised in combination with pectin, a mucoadhesive polymer, which forms a gel in the nasal cavity and from which the active substance is released and absorbed. Hence, even in nasal transmucosal administration, systems based on the use of mucoadhesive substances have been developed to control and increase systemic absorption [38].
Practical Aspects of BTcP TREATMENT
General Premises
Clinical and pharmacological aspects, previously analyzed, are crucial to understand why in clinical practice an optimal therapeutic approach for BTcP should follow different rules and principles from those for BP. The analgesic therapy for BTcP should always be based on an integration of the two therapeutic schemes, for BP and BTcP. On the other hand, in BTcP treatment, several variables may influence the initial choice of the active substance to be used, the possible switch, and the administration route and method as well: characteristics of patient, family and support group (e.g., a professional caregiver), the composition of the care team, the therapeutic setting, and the organizational-management-economic and local regulatory framework, more broadly defined as “context” (Table 4).
BTcP represents a widespread and undertreated clinical problem in cancer patients, even in cases where BP is well controlled by analgesic therapy [2]. It is a common clinical observation, however, that is not always easy to distinguish between variations of BP and BTcP. A clear definition of “adequate control of BP” is not available in scientific literature, although considered by all guidelines as an essential condition to start a specific treatment for BTcP. In our opinion, from a clinical point of view “adequate pain control”, both in BP and in BTcP, means an antalgic effect which decreases the pain intensity to a value ≤2 on a NRS scale. This is true for any value of pain intensity—BP or BTcP—before the start of drug treatment. Therefore, it is advisable that cancer patients with pain be constantly observed and monitored from the initial planning phase of opioid treatment, leaving the decision to the evaluation and experience of each clinician to use rapid-onset opioids (ROOs) or other analgesic systems, such as patient-controlled analgesia (PCA), but suggesting that parenteral medicines be used in the hospital setting only [39–41].
The availability of analgesic medications—opioids in particular—varies among countries due to different registration and marketing policies; some preparations, much used in a country, may not to be available elsewhere. In Italy, for example, morphine, for daily single oral administration, or immediate-release oxycodone is not available. These differences are also present in Italy between regions, and also between local health authorities and hospitals. At present in Italy, only general practitioners (GPs) are authorized to prescribe any approved opioid, thereby ensuring reimbursement of related costs to the patient and family by the Italian National Health System. Lastly in Italy, unlike in other European countries, pain exacerbations, even in a relatively advanced and progressive cancer stage, are often treated with non-steroidal anti-inflammatory drugs (NSAIDs), typically via the intramuscular route, as first-line approach for BTcP [42]. These are inappropriate therapeutic schemes, not recommended in the latest treatment guidelines, but very widely adopted, especially in hospitals. The real impact of NSAID use could be more thoroughly investigated in both qualitative and quantitative terms [43].
Specific Aspects
All variables listed in Table 4 should be considered in the clinical approach to BTcP. This type of “global” approach must be implemented in both phases of the clinical pathway: (a) in the initial phase of selection of the active substance and administration route; and (b) in the subsequent phases, characterized by the achievement of the optimal daily dosage, even in case of switching due to inefficacy, intolerance, or difficulty in administration. It should also be noted that the concept of opioid rotation or opioid switch has developed as part of BP treatment and has been more studied in that area [39] than in BTcP.
The misperception that ROO-administration systems may be superimposed in clinical use as they all release an identical active molecule (fentanyl) is commonly held, and the idea that each product has its own specificity and appropriateness of use has not yet sufficiently disseminated. We need to arrive at a rational choice based on therapy personalization through a careful evaluation of the variables analyzed below, for which a definition as “target BTcP opioid therapy” is suggested.
Possibly the most widespread factors of inappropriateness in treating BTcP currently in Italy are as follows:
-
1.
Lack of clinical recognition of this pain entity, due to an inadequate mode of detection and daily monitoring of pain, even though this is an obligation provided for by art. 7 of Law 38/2010 [44]. This serious shortcoming clearly emerges each year from the Reports that the Minister of Health must communicate to Parliament to comply with art. 11 of Law 38/2010 [45].
-
2.
Use of NSAIDs, especially via the intramuscular route, even for BTcP with repeated daily episodes.
-
3.
Oral use of weak opioids even in the case of intense exacerbations.
-
4.
“Dogmatic” use of oral immediate-release morphine formulations, regardless of the comparative assessment of efficacy for each patient, especially concerning the rapidity of action and the effectiveness profile (efficacy/tolerability ratio) [39, 46–48].
-
5.
Constant use of the same ROO system of fentanyl, among the six ones approved for clinical use in Italy since 2005 (Table 5), regardless of prior assessment of clinical situation and patient preference, and of the potential support offered by patient’s family or caregiver to the therapeutic team.
The causes of these prescriptive behaviors, especially those listed in points 3, 4, and 5 above, are often independent on the level of specific knowledge of clinicians and result from variables outside of their control, such as a non-thorough application of pharmacoeconomic principles by purchasing decision makers. Use of lower cost medications, such as those indicated in points 2, 3, and 4, is preferred, even though it is evident that they do not always represent the optimal treatment in BTcP.
NSAIDs, for example, are associated with a large number of toxic effects [49]. In addition, morphine per os, even in its immediate-release preparations, has an average time required to achieve the peak intensity, significantly more prolonged than ROOs [50]. In the case of BP fluctuations, short-term oral morphine may find indications as a rescue medication, i.e., necessary to adjust the ATC treatment in relation to the circadian pain flares [39]. Its uncritical use, however, in the case of a clear presence of BTcP, exposes patients to some risks: (a) the persistence of intense pain, even for 30 min after onset; (b) non-optimal control of the exacerbation; and (c) pharmacological effects of morphine needlessly longer than the duration of the BTcP episode in relation to the pharmacological and analgesic half-life of the opioid (3–4 h vs. the average BTcP duration of 60–90 min) [50–52].
Variables Involved in the Therapeutic Approach: The Patient
From the patient’s point of view, all points in Table 6 must be carefully taken into account.
In a patient with good cognitive functions and with reasonable motor activity (especially in the upper limbs and hands), the choice of route and system of administration should be based primarily on their preference. This has been shown to be feasible in clinical practice [53]. The patient should be informed and educated about the four routes (gingival fornix, sublingual mucosa, oral mucosa, and nasal mucosa) and the six existing systems [54]. The time dedicated to patient training is balanced by an increased adhesion of the patient to the treatment scheme and by the reduced rates of inefficacy resulting from an incorrect use of the chosen system. It is also clear that systems with easier administration instructions have a greater guarantee of success in patients who are already stressed by daily pain and suffering. In a recent European multicenter study, the oral route was generally the most appreciated [53].
A recent comparative study among three fentanyl ROOs (two oral transmucosal and one nasal), although enrolling a limited number of patients, showed that the use of a product specifically developed for sublingual use was the most appreciated by patients because of its mucoadhesivity, rapid dissolution, and rapid absorption [33].
When using the oral route, patients should be advised that swallowing ROOs before complete dissolution should be avoided, to maximize the bioavailability and not to increase the dosage for a satisfactory analgesic effect. This may not be easy for the patient, as it means keeping one or more tablets without mucoadhesive properties fixed to the oral mucosa for at least 10–15 min, until a complete dissolution [33–35].
The use of the “stick” system, first marketed in Italy in 2005, which sticks to the gingival fornix, may represent the preferred choice for some patients. The same consideration can be made for the two systems that release fentanyl via the nasal route (in aqueous solution or pectin), in which the greater rapidity of action is counterbalanced by the need to have a good level of skill on the part of the patient in the use and loading of the specific devices.
Another important aspect is to assess drug-taking capability during the education and first-prescription phase, recently defined as “accessibility” [33]. This applies particularly in relation to the technical specifications of the packaging for each product. So, in daily use, these apparently simple devices may reduce accessibility. In the context of palliative care, it should be remembered that skills and motor activity of patients may change quickly, for example, in connection with worsening of the asthenia, anorexia–cachexia syndrome, neurological deficit, or with cognitive-relational changes described below.
An additional variable to be borne in mind in the choice of medication is the ability of the patient to maintain the forced postures required (such as maintenance of the supine position) or a possible inability to assume a sitting or semi-sitting position.
Lastly, the methods of titration for the achievement of effective dose must be considered. Methods are specific for each ROO and used correctly in only 42% of the cases treated [3]. In fact, although some recent studies have sought to identify a proportional relationship between the dose of the ATC opioid used for BP and the initial dosage of ROO [55], the general rule is to commence with the lowest available dosage of the chosen ROO and to gradually increase it until the effective dose is reached [52]. Such a procedure—“initial titration”—should be planned when switching to another ROO in the case of a progressive loss of efficacy, after checking that self-administration had been performed correctly. It is quite clear that this assumes a certain importance in the choice by the patient both during instruction and prescription phases of the different dosages used during titration for a quick attainment of the optimal dosage for BTcP control.
The situation is different in the case of: (a) patients with cognitive-relational problems, or (b) with motor activity difficulties, especially in the upper limbs and hands, or difficulty with coordination of the complex buccal motor activity, especially for those subjects characterized by automatic movements of buccal ejection of liquids and solids. Especially in the more advanced stages of the disease, but also in elderly subjects, unconscious motions of sucking or ejection of that introduced by others into their oral cavity may be present.
In the former kind of patients, selection and method of administration become significant and imply an operability that is always “active” for the therapeutic team and more and more “passive” on the part of the patient. If the choice is made not to switch to intravenous bolus administration of opioids, for these patients the most appropriate ROOs are those specifically designed for the sublingual or nasal route (see also the sections “Variables Involved in the Therapeutic Approach: The Care Team” and “Variables Involved in the Therapeutic Approach: Care Setting”). In the latter kind of patients, when difficulties are due to motor activity, an assessment and the preference of the patients should always be requested.
Variables Involved in the Therapeutic Approach: Family and Caregivers
Especially in the context of home care but, sometimes even for assisted patients in residential care homes or hospices, the family unit—and in particular the caregiver—plays an important role in the evaluation of pain and in the interaction with the therapeutic team, but also in drug administration and monitoring of medication efficacy.
As a rule, an important selection criterion in taking charge of home care by a palliative care team is the constant presence of a family caregiver, but often this prerequisite is not fulfilled. The sociological composition of the Italian family, in fact, is increasingly characterized by the presence of “expanded” family units, with a turnover of different relatives in the home during the day; therefore, the presence of non-family caregivers is increasingly widespread, without specific healthcare training and of non-Italian nationality, culture, and language. Increasingly, the patient is assisted by an elderly spouse, who may have problems of reduced autonomy and physical and neuropsychic comorbidities that limit their own ability to support the patient.
Regarding BTcP treatment, the care team should investigate the potential support that can be provided by the family unit or caregiver to formulate a proper therapeutic plan. More than one caregiver is sometimes involved and the situation is therefore more complex, so the course of instruction on the implementation of therapeutic plans becomes more difficult.
When the patient is compliant and sufficiently autonomous, the role of caregiver is more straightforward, limited to checking the correct BTcP medication is self-administered by the patient; the caregiver may improve the accessibility to the medication and verify the exact doses of drug and their efficacy. The caregiver can also be very useful in the titration process aimed at a rapid achievement of the effective ROO dose. The caregiver may also interact with the care team, describing characteristics of pain symptoms, thus becoming a valid “third-party” observation.
The role of professional caregivers or family members increases with the worsening of the disease and the progressive loss of patient autonomy. If an active support role is not provided, the risk is an incorrect pain management during the day, with a consequent request for “unscheduled” home visits by the care teams (e.g., GP, palliative care team, or continuity of care service). In some cases, especially at night or in weekends, the uncontrolled pain crises can lead to unnecessary and inappropriate access to the Emergency Health Network (e.g., to the hospital emergency or the community emergency service). It is recommended that, even in the initial BTcP treatment prescription, physicians and nurses take into proper account some important variables (Table 7).
Comparative studies on currently available products and their administration by caregivers or family members, and on their preferences and assessments, are lacking, but it can be argued that the initial choice of an effective product for BTcP treatment can be based on three main characteristics:
-
1.
Ease of use;
-
2.
A proper use of drug by the patient;
-
3.
The time necessary to check the successful absorption of the active substance.
The non-mandatory use of specific delivery devices and the rapidity of drug dissolution are two variables that, on a case-by-case basis, should be integrated, in a matrix model, with the other three characteristics described above (Table 8).
Variables Involved in the Therapeutic Approach: The Care Team
The definition of health care team in the context of palliative care or pain therapy is very broad, especially due to the absence of national and regional reference standards. Differences are observed in reference to each setting (e.g., the hospice), among different settings, and, finally, in reference to the so-called “intensity of care”, the number of direct accesses to the patient by team members in relation to a pre-defined unit of time (e.g., number of days with at least one home visit in relation to the total number of days of patient management) [56].
The composition of the care team and the time available for direct and indirect activities assigned to each operator are important variables to ensure an adequate response to the needs of patients/families. Their importance grows when, especially in the early stages, the diagnostic and therapeutic interventions require maximum attention to detail and a high willingness to provide explanations and correct instructions to the patient and their families (caregivers). In the case of BTcP treatment, the patient and their caregiver should be trained as quickly as possible in the basic principles (e.g., proper use of medication, titration, repeat times of administration, etc.), essential for the therapeutic success.
Other important differentiating aspects in the approach to BTcP are the seriousness in the training and experiential pathway and the attitude to the innovation and the introduction of new pharmaceutical preparations in clinical use.
In the training process, the information transmitted “on the job” by one operator to another in the daily debate and audit is very important and often independent of the classical modes of training/learning (e.g., frontal lessons). In this case also, there are no specific studies on the attitudes and preferences of the care team members in relation to the different settings and treatment options.
From a theoretical point of view, it can be possible that, when all treatment options are available, the care team would choose systems easier to use, that require fewer instructions, have fewer limitations, less observation of the patient, and a greater safety level.
Variables Involved in the Therapeutic Approach: Care Setting
BTcP could occur in every care setting which provides assistance to cancer patients in the advanced stages of therapy: outpatient care, day hospital, inpatient care in a hospital specialist unit or hospice, residential care home, or at home. In the care settings in which a health and social care team is constantly present and specifically trained in pain therapy and palliative care, the variables related to accessibility to the product become less important, unless the care team delegates administration of the medication to the patient or caregiver.
In each setting, in the choice of BTcP treatment, the referring clinician and care team should consider all the variables described, relating to the patient and family member/caregiver.
The ease of use is a “transversal” variable in the training and prescription process in all care settings. Settings characterized by a greater intensity of care can adopt more complex treatments including intravenous administration of bolus of short-term opioids or infusion systems for PCA methods.
Variables Involved in the Therapeutic Approach: The Context
The analysis of the specific Italian context in the care framework offers an opportunity for some final considerations which may help explain why, compared to other European countries, BTcP in Italy is still probably insufficiently and often improperly treated. According to article 10 of Law 38/2010 [44], GPs can prescribe all ROO medications currently approved for sale in Italy; this is not the rule for physicians operating within health and social welfare facilities. In some regions, medical specialists, including palliativists and algologists, are not authorized to directly prescribe medications so that the medication costs can be reimbursed by the regional health system, and they may prescribe opioids only through the specific personal prescription book for psychoactive medications (still in use, despite the changes introduced by article 10 of Law 38/2010 [44]) or through their own personal prescription book. In these latter two cases, the patient and/or family member must purchase the product in the pharmacy, having no right to reimbursement by the Italian National Health Service (NHS).
In some situations, mostly in the public or private non-profit “hospital-at-home” model, present only in certain regions (e.g., in Lombardy), the care team can supply medications (including ROOs) directly to the patient at home, provided that the drugs are included in the regional and/or local pharmacy formulary, or purchased from the facility to which the palliative care unit belongs.
A first consequence of these limitations is that, where the specialist prescription is not direct but presented as a “therapeutic recommendation”, the patient must have the medication or medications “registered” by the GP in the Italian NHS’s prescription book. This is not always automatic since each practitioner has their own base of scientific opinions, knowledge, and experiences. Following the Law 38/2010 and related training projects, GPs have acquired a specific cultural basis in treating pain [57].
Furthermore, only a minority of Italian regions and local health authorities have approved their own pharmaceutical formulary; the mechanisms of authorization for the use of medications, especially for hospitalized patients, are markedly diversified among regions and even within the same region. Some regions are characterized by a more “centralized” medication policy management, sometimes based on the opinion of regional technical bodies established “ad hoc”. In others, decision makers are more “peripheral”, consisting of the hospital pharmacist or the local health authority, who can strongly influence the acquisition/availability of medicines. In the case of pharmaceutical products containing the same active substance, such as fentanyl ROOs, the trend could be an underestimation of the specificities of each product, considering them all equal. The hospital clinician, unlike the GP, still does not have all pharmaceutical products, generally having available the products which were first on the market, or those at lowest cost. In some situations, the only possibility of BTcP treatment for specialists is the use of short-term morphine per os.
Even where a region clearly indicates, through a specific legislative measure, that the specialist must have at disposal all the active substances and products authorized for clinical use for pain treatment, the opposition to the application at the peripheral level is strong and varies between one hospital, and one local health authority, and another [58].
On the other hand, the technical bodies within each hospital and each local health authority since 2001, the Committees for the Pain-Free Hospital (COSD), subsequently redefined by article 6 of the Law 38/2010 as the Committees for the Pain-Free Hospital/Community (COTSD), with a few exceptions, have not been able to introduce elements useful to overcome this critical situation. This is because they have been established in a minority of registered healthcare facilities and, where present, their functioning has not been continuous and they have no real powers of changing the actual situation.
Finally, it is clear that the social context and the degree of social and “collective” sensitivity to the issue of pain and suffering are elements which can facilitate or create an obstacle to the treatment of BTcP.
Brief Recommendations in the Use of Medications in BTcP
The main points contained in the article are briefly listed below:
-
1.
Circadian exacerbations of pain should be carefully monitored, differentiating, if possible, between changes of BP, end-of-dose effect, and BTcP.
-
2.
BTcP should be monitored in all care contexts in clinical practice.
-
3.
Each care facility must have all the medications and products approved for use in BTcP; the COSD/COTSD must make all efforts to achieve this result.
-
4.
Medications for treatment of BTcP are not automatically interchangeable with one other, even if they contain the same active substance.
-
5.
Each practitioner must know the specific characteristics of each medication and the differences in pharmacological properties and possible limitations in clinical practice.
-
6.
Each practitioner must know the specifics for titration and the repeatability of administration (the so-called lock-out period between one administration and the other) for each medication used for BTcP treatment.
-
7.
Each practitioner must know the technical specifics for accessibility (referring to the packaging) and delivery of the medications useful for treatment of BTcP.
-
8.
Physicians and nurses working as a team must know the prescriptive methods of the medications useful in treatment of BTcP.
-
9.
Before choosing the ROO, particular attention must be given to gaining greater knowledge of the variables concerning the patient and their family unit/caregivers, taking into account the progressive loss of autonomy and/or cognitive-relational functionality of the patient.
-
10.
When it is decided to commence BTcP therapy and whenever its therapy is changed, special attention must be paid to clearly and sufficiently training patient and family member/caregiver.
-
11.
The patient must be treated effectively with major opioids for BP before introducing ROOs for BTcP.
-
12.
ROOs, at the present state of knowledge, must not be used in treatment of BTcP secondary to a non-cancer pathology.
References
Hagen NA, Biondo P, Stiles C. Assessment and management of breakthrough pain in cancer patients: current approaches and emerging research. Curr Pain Headache Rep. 2008;12:241–8.
Davies A, Dickman A, Reid C, Science Committee of the Association for Palliative Medicine of Great Britain and Ireland, et al. The management of cancer-related breakthrough pain: recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur J Pain. 2009;13:331–8.
Corli C, Pizzuto M, OICP Research Group. Per qualche dolore in meno… Capire e trattare il breakthrough cancer pain. Roma: CIC Edizioni Internazionali; 2011.
Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain. 1990;41:273–81.
Agency for Healthcare Research and Quality. Management of cancer pain summary. 2001; evidence report/technology assessment number 35, AHRQ publication number 01-E033.
Walsh D. Pharmacological management of cancer pain. Semin Oncol. 2000;27:45–63.
Walsh D, Rivera NI, Davis MP, et al. Strategies for pain management: Cleveland Clinic Foundation guidelines for opioid dosing for cancer pain. Support Cancer Ther. 2004;1:157–64.
Zeppetella G. Opioids and breakthrough pain. In: Fifth Bristol Opioid conference. Bristol University Press; 2010.
Gomez-Batiste X, Madrid F, Moreno F, et al. Breakthrough cancer pain: prevalence and characteristics in patients in Catalonia, Spain. J Pain Symptom Manag. 2002;24:45–52.
Hwang SS, Chang VT, Kasimis B. Cancer breakthrough pain characteristics and responses to treatment at a VA medical center. Pain. 2003;101:55–64.
Caraceni A, Martini C, Zecca E, et al. Working Group of an IASP Task Force on Cancer Pain. Breakthrough pain characteristics and syndromes in patients with cancer pain. An international survey. Palliat Med. 2004;18:177–83.
Svendsen KB, Andersen S, Arnason S, et al. Breakthrough pain in malignant and non-malignant diseases: a review of prevalence, characteristics and mechanisms. Eur J Pain. 2005;9:195–206.
Zeppetella G. Breakthrough pain in cancer patients. Clin Oncol (R Coll Radiol). 2011;23:393–8.
Dickenson A. The neurobiology of chronic pain states. Anaesth Intensive Care Med. 2013;14:484–7.
Wordliczek J, Zajaczkowska R. Mechanisms in cancer pain. In: Hanna M, Zylicz Z, editors. Cancer pain. London: Springer; 2013.
Cervero F, Meyer RA, Campbell JN. A psychophysical study of secondary hyperalgesia: evidence for increased pain to input from nociceptors. Pain. 1994;58:21–8.
Caraceni A, Bertetto O, Labianca R, et al. Breakthrough/Episodic Pain Italian Study Group. Episodic (breakthrough) pain prevalence in a population of cancer pain patients. Comparison of clinical diagnoses with the QUDEI–Italian questionnaire for intense episodic pain. J Pain Symptom Manag. 2012;43:833–41.
Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–15.
Campisi G, Paderni C, Saccone R, et al. Human buccal mucosa as an innovative site of drug delivery. Curr Pharm Des. 2010;16:641–52.
Narang N, Sharma J. Sublingual mucosa as a route for systemic drug delivery. Int J Pharm Pharm Sci. 2011;3(Suppl 2):18–22.
Shojaei AH. Buccal mucosa as a route for systemic drug delivery: a review. J Pharm Pharm Sci. 1998;1:15–30.
Madhav NV, Shakya AK, Shakya P, Singh K. Orotransmucosal drug delivery systems: a review. J Control Release. 2009;140:2–11.
Bartlett JA, van der Voort Maarschalk K. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. 2012;13:1110–5.
Davis MP, Glare PA, Hardy J, Quigley C, editors. Opioids in cancer pain. 2nd ed. Oxford: Oxford University Press; 2009.
Smith HS. Considerations in selecting rapid-onset opioids for the management of breakthrough pain. J Pain Res. 2013;6:189–200.
Farrar JT, Cleary J, Rauck R, et al. Oral transmucosal fentanyl citrate: randomized, double-blinded, placebo-controlled trial for treatment of breakthrough pain in cancer patients. J Natl Cancer Inst. 1998;90:611–6.
Portenoy RK, Taylor D, Messina J, Tremmel L. A randomized, placebo-controlled study of fentanyl buccal tablet for breakthrough pain in opioid-treated patients with cancer. Clin J Pain. 2006;22:805–11.
Rauck R, North J, Gever LN, et al. Fentanyl buccal soluble film (FBSF) for breakthrough pain in patients with cancer: a randomized, double-blind, placebo-controlled study. Ann Oncol. 2010;21:1308–14.
Rauck RL, Tark M, Reyes E, et al. Efficacy and long-term tolerability of sublingual fentanyl orally disintegrating tablet in the treatment of breakthrough cancer pain. Curr Med Res Opin. 2009;25:2877–85.
Rauck R, Reynolds L, Geach J, et al. Efficacy and safety of fentanyl sublingual spray for the treatment of breakthrough cancer pain: a randomized, double-blind, placebo-controlled study. Curr Med Res Opin. 2012;28:859–70.
Kress HG, Oronska A, Kaczmarek Z, et al. Efficacy and tolerability of intranasal fentanyl spray 50 to 200 microg for breakthrough pain in patients with cancer: a phase III, multinational, randomized, double-blind, placebo-controlled, crossover trial with a 10-month, open-label extension treatment period. Clin Ther. 2009;31:1177–91.
Portenoy RK, Burton AW, Gabrail N, Taylor D. A multicenter, placebo-controlled, double-blind, multiple-crossover study of fentanyl pectin nasal spray (FPNS) in the treatment of breakthrough cancer pain. Pain. 2010;151:617–24.
Moore N, Darwish M, Amores X, Schneid H. A review of the pharmacokinetic profile of transmucosal fentanyl formulations. Curr Med Res Opin. 2012;28:1781–90.
Darwish M, Kirby M, Robertson P Jr, et al. Comparison of equivalent doses of fentanyl buccal tablets and arteriovenous differences in fentanyl pharmacokinetics. Clin Pharmacokinet. 2006;45:843–50.
Darwish M, Kirby M, Jiang JG, et al. Bioequivalence following buccal and sublingual placement of fentanyl buccal tablet 400 microg in healthy subjects. Clin Drug Investig. 2008;28:1–7.
Woodcock J, Khan M, Yu LX. Withdrawal of generic budeprion for nonbioequivalence. N Engl J Med. 2012;367:2463–5.
Bredenberg S, Duberg M, Lennernäs B, et al. In vitro and in vivo evaluation of a new sublingual tablet system for rapid oromucosal absorption using fentanyl citrate as the active substance. Eur J Pharm Sci. 2003;20:327–34.
Grassin-Delyle S, Buenestado A, Naline E, et al. Intranasal drug delivery: an efficient and non-invasive route for systemic administration: focus on opioids. Pharmacol Ther. 2012;134:366–79.
Caraceni A, Hanks G, Kaasa S, European Association for Palliative Care (EAPC), et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13:e58–68.
Mercadante S, Villari P, Ferrera P, et al. Transmucosal fentanyl vs intravenous morphine in doses proportional to basal opioid regimen for episodic-breakthrough pain. Br J Cancer. 2007;96:1828–33.
Mercadante S, Villari P, Ferrera P, et al. Safety and effectiveness of intravenous morphine for episodic (breakthrough) pain using a fixed ratio with the oral daily morphine dose. J Pain Symptom Manag. 2004;27:352–9.
Pain therapy: Fans in Italy are 4 times more expensive than opioids. Available from: http://www.fondazioneisal.it/en/pubblicazioni/weblog/178-cura-del-dolore-in-italia-la-spesa-per-i-fans-e-4-volte-quella-per-gli-oppiacei.html. Accessed February 25, 2014.
Mercadante S. Pharmacotherapy for breakthrough cancer pain. Drugs. 2012;72:181–90.
Italian Law n. 38 of 2010. Available from: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1487_allegato.pdf. Accessed February 25, 2014.
Palliative Care and Pain Therapy—Reports to Italian Parliament. Available from: http://www.salute.gov.it/portale/temi/p2_6.jsp?lingua=italiano&id=3761&area=curePalliativeTerapiaDolore&menu=legge. Accessed February 25, 2014.
Opioids in palliative care: safe and effective prescribing of strong opioids for pain in palliative care of adults. NICE Clinical Guidelines, No. 140. National Collaborating Centre for Cancer (UK). Cardiff (UK): National Collaborating Centre for Cancer (UK); 2012 May.
Vissers D, Stam W, Nolte T, Lenre M, Jansen J. Efficacy of intranasal fentanyl spray versus other opioids for breakthrough pain in cancer. Curr Med Res Opin. 2010;26:1037–45.
Fallon M, Reale C, Davies A, et al. Efficacy and safety of fentanyl pectin nasal spray compared with immediate-release morphine sulfate tablets in the treatment of breakthrough cancer pain: a multicenter, randomized, controlled, double-blind, double-dummy multiple-crossover study. J Support Oncol. 2011;9:224–31.
Bhala N, Emberson J, Merhi A, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382:769–79.
Zeppetella G. Dynamics of breakthrough pain vs. pharmacokinetics of oral morphine: implications for management. Eur J Cancer Care (Engl). 2009;18:331–7.
Coluzzi PH, Schwartzberg L, Conroy JD, et al. Breakthrough cancer pain: a randomized trial comparing oral transmucosal fentanyl citrate (OTFC) and morphine sulfate immediate release (MSIR). Pain. 2001;91:123–30.
Bornemann-Cimenti H, Wejbora M, Szilagyi IS, Sandner-Kiesling A. Fentanyl for the treatment of tumor-related breakthrough pain. Dtsch Arztebl Int. 2013;110:271–7.
Davies A, Zeppetella G, Andersen S, et al. Multi-centre European study of breakthrough cancer pain: pain characteristics and patient perceptions of current and potential management strategies. Eur J Pain. 2011;15:756–63.
England R, Manderson C, Zadora-Chrzastowska S, et al. How practical are transmucosal fentanyl products for breakthrough cancer pain? Novel use of placebo formulations to survey user opinion. BMJ Support Palliat Care. 2011;1:349–51.
Mercadante S, Ferrera P, Adile C, Casuccio A. Fentanyl buccal tablets for breakthrough pain in highly tolerant cancer patients: preliminary data on the proportionality between breakthrough pain dose and background dose. J Pain Symptom Manag. 2011;42:464–9.
Zucco F. Gli Hospice in Italia 2010: seconda rilevazione ufficiale. Bologna: ed. Bononia University Press, Settembre 2010, pp 1–420.
Fanelli C, Ventriglia G (eds). Il dolore cronico in medicina generale. Ministero della Salute 2010.
Le 10 Raccomandazioni. Decreto Direttore Generale Sanità, Regione Lombardia, n. 23454, 30/12/2004 con Oggetto: Determinazioni per la costituzione del Comitato Ospedale Senza Dolore (COSD) presso le Strutture Sanitarie di ricovero e cura e adozione del “Manuale Applicativo per la realizzazione dell’Ospedale senza Dolore”. pag. 22. Available from: http://www.fedcp.org/pdf/ospedale_senza_dolore.pdf. Accessed February 25, 2014.
Acknowledgments
Editorial assistance in the preparation of this manuscript was provided by Brunilde Iovene, an independent medical writer, and by Mary Hines of Springer Healthcare Communications. Support for this assistance was funded by ProStrakan Srl. No other funding or sponsorship was received for this study or publication of this article. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Conflict of interest
Cesare Bonezzi has received research grants from ProStrakan Srl. Diego Fornasari has received research grants from ProStrakan Srl. Furio Zucco has received research grants from ProStrakan Srl.
Compliance with ethics guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zucco, F., Bonezzi, C. & Fornasari, D. Breakthrough Cancer Pain (BTcP): a Synthesis of Taxonomy, Pathogenesis, Therapy, and Good Clinical Practice in Adult Patients in Italy. Adv Ther 31, 657–682 (2014). https://doi.org/10.1007/s12325-014-0130-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-014-0130-z