Abstract
BCR-ABL-negative primary myelofibrosis (PMF) shows non-specific karyotype abnormalities in up to 30 % of patients, e.g. chromosome 7 defects, which are associated with an adverse prognosis. The impact of chromosome 7 aberrations on cell pathobiology is not yet known. We speculated that chromosome 7q-encoded regulatory microRNA might be one possible basis of instable disease. JAK2V617F-positive cases of PMF with 7q defects (n = 4) were compared with PMF with non-7 aberrations (n = 3), PMF without karyotype aberrations (n = 3) and with a pool of three non-neoplastic controls. The microRNA expression profile of bone marrow cells was analysed with real-time PCR arrays. No 7q-related profile was found but, independent of the underlying karyotype, PMF cases show higher levels of 10q-encoded miR-146b. Re-evaluation in a second cohort (n = 50) confirmed miR-146b overexpression in fibrotic stage PMF. On the transcript level, no negative regulation of potential miR-146b targets could be found. In summary, no link between cytogenetic alterations and microRNA expression could be verified in PMF with 7q defects but 10q-encoded miR-146b overexpression was found to be associated with fibrosis.
Similar content being viewed by others
Introduction
Philadelphia chromosome-negative myeloproliferative neoplasms (Ph− MPN) can acquire cytogenetic defects [1]. In particular, in primary myelofibrosis (PMF), up to 30 % of patients have an aberrant somatic karyotype in their neoplastic haematopoiesis, while in non-fibrosing polycythemia vera (PV) and essential thrombocythemia (ET), the frequency is lower. Recurrent defects are +8, +9, del(13q) and del(20q), but these chromosomal aberrations are not specific for PMF [1, 2]. They can be also found in other Ph− MPN entities and as secondary alterations in Ph+ chronic myelogenous leukaemia (CML), in myelodysplastic syndromes (MDS) and acute myeloid leukaemias (AML) [1–4]. It has been shown that the presence of similar types of karyotype anomalies in MDS and PMF, in particular −7/del(7q), is associated with an adverse prognosis (lower survival rates and a higher rate of secondary AML transformation) [4–8]. It is not fully understood why these chromosome 7 aberrations have such an impact on cell pathobiology [3, 9]. Besides protein-coding genes, hundreds of microRNA genes are encoded on autosomal chromosomes and the X chromosome [10–12]. Precursor transcripts (100–150 nucleotides in length) from these genes are processed to 20–25-nucleotide short RNA molecules, the biologically active mature microRNAs [10–12]. These RNA molecules bind in a semi-complementary fashion to the 3′-untranslated region (3′-UTR) of mRNA molecules which inhibits protein translation and therefore represents a complex regulatory system of cell homeostasis in normal and neoplastic tissues [10–12]. The role of microRNAs appears to be more a modulation of mRNA/protein translation and not a complete suppression [10–12].
MicroRNA expression analyses have been performed in chronic stage MPN, including PMF, but have not been correlated with underlying chromosomal defects [13–17]. At least in one post-PMF AML case with t(12;18)(p13.2;q12.3), it has been demonstrated that expression of 18q12.3-encoded miR-4319 is decreased [18]. In MDS with del(5q), chronic lymphocytic B cell leukaemia with del(13q) and splenic marginal zone lymphoma with del(7q32) there are hints that microRNAs which are encoded on the deleted chromosome segment can be involved in mediation of disease phenotypes [19–21]. In contrast to monogenic neoplasms which are based on one single or at least one major defect, e.g. CML, no haematopoietic or solid organ neoplasm has been identified in which one single microRNA is the disease-causing aberration [10–12]. Therefore, a shift of the total microRNA expression profile rather than deregulation of one single microRNA is more likely to have a pathobiological effect on cell homeostasis in MPN. In this analysis, we aimed to evaluate whether such a shift could be related to chromosome 7q defects in PMF.
Materials and methods
Study groups
Study group I (microRNA profiling): PMF samples for screening of karyotype-associated microRNA profiles represent a relatively homogeneous group which mainly differ by their karyotypes (n = 10). All cases had advanced stage fibrosis and osteosclerosis (MF3), myeloblasts were <5 % in all cases, and all cases were JAK2V617F-positive. DNA extraction and PCR-based JAK2V617F mutation analysis with a pyrosequencer device were performed as previously described [22]. The microRNA expression profile was tested in PMF without karyotype aberrations (n = 3), PMF with isolated del(7q) (n = 2), translocations affecting 7q (n = 2), and PMF with non-7 aberrations (n = 3). As previously published, JAK2V617F-negative PMF with chromosome 7 defects could not be identified in our cohort [2]. For control purposes, a pool of three age-matched cases with non-neoplastic haematopoiesis was analysed (Table 1, supplementary Table 1).
Study group II (re-evaluation of single microRNA and target mRNA): Re-evaluation was performed for single gene transcripts in a second cohort of MPN and controls (n = 50). Each 10 pre-fibrotic PMF (MF0, all with normal karyotypes), fibrotic PMF (MF2-3, 7/10 with normal karyotype, 3/10 not tested), PV (MF0, two with non-7 defects, all others normal karyotypes), ET (MF0, all normal karyotypes) and non-neoplastic controls were analysed (Table 1).
All samples, including controls, were collected as part of standard clinical care for evaluation of bone marrow status. Bone marrow trephines were formalin-fixed and paraffin-embedded (FFPE) and were retrieved from the tissue registry of the Institute of Pathology, Hannover Medical School. The study was approved by the local ethics committee.
MicroRNA profiles in PMF with cytogenetic aberrations
RNA was isolated from bone marrow cells by proteinase K digestion and phenol/chloroform extraction as described [23, 24]. MicroRNA profiling was performed by using 3 μl/1 ng of the RNA solution for reverse transcription in 7.5 μl reactions (TaqMan Megaplex Pools with Preamplification, Applied Biosystems, Foster City, CA, USA). Similar to our previous study [24], as a non-neoplastic reference, RNA from three cases with normal haematopoiesis was pooled. TaqMan Array Human MicroRNA Panel A which includes 378 microRNA and three endogenous control genes (small nucleolar RNA, C/D box 48/RNU48 which is encoded on segment 6p21.33; RNU44, 1q25.1; RNA, U6 small nuclear 1/U6, 15q23) were loaded with a mixture of 1 part diluted PreAmp product (1:4), 9 parts H2O and 50 parts Universal PCR Master Mix (Applied Biosystems). The real-time PCR runs were performed on a 7900HT Fast Real-time PCR system (Applied Biosystems).
Re-evaluation of miR-146b and potential targets in MPN
Bone marrow-derived RNA was used in the second cohort for measuring the expression of the 10q24.32-encoded miR-146b (endogenous control RNU48). In addition, two potential miR-146b target molecules, matrix metallopeptidase 16 (MMP16, encoded on segment 8q21.3) and tissue inhibitor of metallopeptidases 4 (TIMP4, 3p25) and endogenous control gene polymerase (RNA) II (DNA-directed) polypeptide A, 220 kDa (POLR2A, 17p31.1) were evaluated from the same RNA (TaqMan Assays, ABI PRISM 7500 Sequence Detector, both Applied Biosystems) [23, 24].
Data analysis
CT values were converted into ΔCT values (endogenous control gene: RNU48 for microRNA and POLR2A for mRNA) and further into 2-ΔCT (normalized against RNU48 and POLR2A, respectively). As a pre-screening, expression levels of cohort I were sorted according to the levels of non-neoplastic control pool data and the mean values of each of the three cytogenetic PMF groups. Arbitrary, mean fold-changes of >2 were considered as up- and down- regulation. PMF groups were compared to each other with the non-parametric Kruskal-Wallis test followed by Dunn’s post test and exact p values were determined with the two-tailed Mann–Whitney test (Prism 5.0, GraphPad Software, San Diego, CA, USA). Cluster analysis of microRNA profiles was performed with the Qlucore Omics Explorer 2.2 (Qlucore AB, Lund, Sweden).
In cohort II, transcript expression levels of single genes were compared with the Kruskal-Wallis and Dunn’s post test followed by the two-tailed Mann-Whitney test (Prism 5.0). Correlation analysis of miR-146b/TIMP4 levels was performed with Spearman’s test (Prism 5.0). P values <0.05 were considered statistically significant.
Results
No chromosome 7q-related microRNA expression profile in PMF
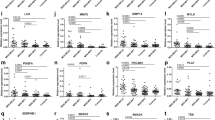
The particular underlying chromosomal defects were correlated with the expression of microRNAs which are encoded on the chromosome segments involved. Chromosome 7q-encoded microRNA, such as miR-25 (7q22.1), miR-93 (7q22.1), miR-106b (7q22.1), miR-96 (7q32.2), miR-182 (7q32.2), miR-183 (7q32.2), miR-335 (7q32.2) and miR-29a (7q32.3), were not specifically deregulated in PMF with 7q21-35 defects (Fig. 1, supplementary Table 2). Cluster analyses revealed an expression profile which could not discriminate PMF with different karyotype aberrations and normal karyotypes.
No deregulation of chromosome 7-encoded microRNA but increased expression of miR-146b in fibrotic stage PMF. a-d Four examples of 7q-encoded microRNA are depicted and show no tendency for a deregulation in PMF with 7q defects. Mean and standard deviation are depicted by bars. The pool of three control cases represents normal haematopoiesis (dot with bar). In the screening cohort I, all PMF cases were in advanced fibrotic stages. e-g MiR-146a was moderately increased in some PMF while its paralogue miR-146b showed a tendency for high levels in fibrotic PMF. Re-evaluation (cohort II) confirmed that fibrotic stage PMF cases express high or very high levels of miR-146b. The difference was significant: PMF MF2-3 (mean relative expression level 11.85, standard deviation ±11.89) versus PMF MF0 (3.21 ± 1.33; p = 0.02*), PV (2.07 ± 1.16; p = 0.002**), ET (1.91 ± 1.22; p = 0.0007***) and controls (0.70 ± 0.43; p < 0.0001***). Furthermore, PMF MF0 (p < 0.0001***), PV (p = 0.0007***) and ET (p = 0.0089***) had significantly higher levels than controls. h TIMP4 was increased in fibrotic stage PMF (not significant) and no negative miR-146b/TIMP4 correlation was found (Pearson r = 0.4). Abbreviations: PMF MF3 cases with 7q defects (PMF, 7q), with non-7q defects (PMF, non-7q) and with normal karyotypes (PMF, NK), pool of three non-neoplastic controls with normal karyotypes, cohort I (C, pool), grade of fibrosis in fibrotic PMF, pre-fibrotic PMF, PV and ET without fibrosis, cohort II (MF 0-3), non-neoplastic control cases, cohort II (C)
Compared with normal bone marrow cells, PMF with chromosome 7q defects but also PMF with non-7q defects showed a tendency towards lower levels of the miR-17 precursor family, which includes miR-17 (encoded on chromosome segment 13q31.3), miR-20a (13q31.3), miR-20b (Xq26.2), miR-106a (Xq26.2), miR-106b (7q22.1) and miR-93 (7q22.1). Compared with the control pool, PMF with normal karyotypes mainly had similar levels of miR-17 precursor family members (supplementary Table 2). Therefore, the cytogenetic defects under investigation have no direct effect on the microRNA expression profile in PMF with chromosome 7q defects.
Increased miR-146b expression in PMF is independent from chromosomal defects
In non-neoplastic bone marrow cells, a total of 24 microRNAs were expressed with a relative level of ≥1 (supplementary Table 2). Similar to previous results [11, 12], the microRNA with the highest expression level was miR-223.
Independent of the chromosomal defects, in general, in PMF, the 17q22-encoded miR-142-3p and miR-208b (14q11.2) showed lower expression levels as well as higher levels of miR-146b (10q24.32) (Fig. 1 supplementary Table 2). We selected miR-146b for re-evaluation in the second cohort because this microRNA is linked to the regulation of fibrosis-associated factors, such as MMP16 and TIMP4 [25, 26]. Therefore, the transcript expressions of these two genes were also analysed. We found that a subfraction of fibrotic stage PMF overexpressed miR-146b (versus non-neoplastic controls p < 0.0001; versus pre-fibrotic PMF, PV and ET each p ≤ 0.02). MMP16 transcripts could not be detected. TIMP4 was expressed at higher levels in fibrotic stage PMF, but the differences were not significant (Fig. 1). No negative correlation between miR-146b and TIMP4 was found, indicating no direct negative regulation.
Discussion
Based on previous results on haematopoietic neoplasms, we hypothesized that a deletion of a chromosomal region could lead to decreased gene expression of encoded microRNA. Low grade B cell lymphomas with del(7q32) show lower levels of 7q32-encoded miR-29a and miR-29b-1 [21]. In contrast to lymphomas, we could not find a direct effect of chromosome 7q defects and deregulated expression of 7q-encoded microRNA in PMF. It has been shown in MDS that the expression of microRNAs and protein-coding mRNA transcripts can be related to chromosome 7 or del(5q) defects, but the corresponding genes are mainly not 7- or 5q-encoded [7, 23, 24, 27–29]. For example, there are new data that show that MDS-del(5q) shows aberrant expression profiles of several 14q-encoded microRNAs [29]. This indicates that microRNA expression could be transregulated, e.g. hypermethylation of a 9p-encoded gene is found more often in MDS with −7 [7] or in t(9;22)(q34;q11)-positive CML, the chromosome 17q-encoded miR-451 can bind to BCR-ABL fusion mRNA while BCR-ABL protein suppresses transcription of miR-451 [17].
Although we could not prove our hypothesis of a chromosome 7q-related microRNA expression profile in PMF, we noted that, independent of the underlying karyotype, fibrotic stage PMF showed higher levels of 10q-encoded miR-146b. In a second cohort, we could confirm an association of fibrosis and increased miR-146b levels. Furthermore, a recent study from Korea also revealed increased miR-146b expression in PMF bone marrow cells [30]. In PMF-derived CD34+ cells, miR-146b is also increased while the megakaryocytic differentiation of PMF-derived CD34+ cells is paralleled by increase of 21q-encoded miR-155 levels [15]. Previously, we could show that PMF bone marrow-derived megakaryocytes have an increased miR-146b expression in situ but not miR-155 [14]. While bone marrow megakaryocytes and CD34+ cells express miR-146b, this microRNA is not increased in the peripheral blood of PMF patients [31]. This could indicate that miR-146b plays a role in the modulation of the bone marrow microenvironment, i.e. in megakaryocytic cell homeostasis, and not in circulating leukocytes and platelets. In principle, increased miR-146b expression could contribute to fibrosis, e.g. by interfering in the MMP16/MMP2 axis or by inhibiting TIMP4 and SMAD family member 4 (SMAD4) transcripts [25, 26, 32]. Secreted MMP16 activates MMP2 by cleavage in the extracellular matrix and MMP2 degrades type IV collagen and increased miR-146b can alter these protein interactions [25]. We could show that MMP2 is increased and not decreased in fibrotic stage PMF [33], but in this current analysis, we could not detect MMP16 transcripts in MPN and non-neoplastic haematopoiesis. Therefore, we found no evidence of an miR-146b/MMP16 deregulation. SMAD4 is an intracellular factor which is related to the transforming growth factor (TGF) beta-induced signalling. We could previously show that the TGF beta type II receptor (TGFR2) is increased in advanced stage PMF while SMAD4 is not deregulated on the transcript level [33] which makes a relevant miR-146b/SMAD4 deregulation unlikely. It has been shown that miR-146b/TIMP4 regulation is associated with atrial fibrosis [26], and we therefore evaluated whether this molecular mechanism could be found also in fibrotic stage PMF. Although we found no direct miR-146b/TIMP4 regulation on the transcript level in PMF, a modulation of the mRNA/protein translation could be possible [26]. Therefore, a subfraction of fibrotic stage PMF shows an increased miR-146b expression but the major fibrosis-associated molecular defect remains to be identified.
In summary, in PMF bone marrow cells, the expression of 7q-encoded microRNAs is not related to defects of chromosome segment 7q while microRNAs, such as miR-146b, which are encoded on other chromosome segments, can be deregulated.
References
Swerdlow SH, Campo C, Harris NL (eds) (2008) WHO classification of tumours of haematopoietic and lymphoid tissues. IARC press, Lyon
Hauck G, Jonigk D, Göhring G, Kreipe H, Hussein K (2013) Myelofibrosis in Philadelphia chromosome-negative myeloproliferative neoplasms is associated with aberrant karyotypes. Cancer Genet 206:116–123
Haase D (2008) Cytogenetic features in myelodysplastic syndromes. Ann Hematol 87:515–526
Tefferi A, Sirhan S, Sun Y, Lasho T, Finke CM, Weisberger J, Bale S, Compton J, LeDuc CA, Pardanani A, Thorland EC, Shevchenko Y, Grodman M, Chung WK (2009) Oligonucleotide array CGH studies in myeloproliferative neoplasms: comparison with JAK2V617F mutational status and conventional chromosome analysis. Leuk Res 33:662–624
Li B, Xu J, Li C, Gale RP, Xu Z, Qin T, Zhang Y, Huang G, Fang L, Zhang H, Pan L, Hu N, Qu S, Xiao Z (2014) Cytogenetic studies and their prognostic contribution in 565 Chinese patients with primary myelofibrosis. Am J Hematol 89:1043–10436
Guglielmelli P, Lasho TL, Rotunno G, Score J, Mannarelli C, Pancrazzi A, Biamonte F, Pardanani A, Zoi K, Reiter A, Duncombe A, Fanelli T, Pietra D, Rumi E, Finke C, Gangat N, Ketterling RP, Knudson RA, Hanson CA, Bosi A, Pereira A, Manfredini R, Cervantes F, Barosi G, Cazzola M, Cross NC, Vannucchi AM, Tefferi A (2014) The number of prognostically detrimental mutations and prognosis in primary myelofibrosis: an international study of 797 patients. Leukemia 28:1804–1810
Christiansen DH, Andersen MK, Pedersen-Bjergaard J (2003) Methylation of p15INK4B is common, is associated with deletion of genes on chromosome arm 7q and predicts a poor prognosis in therapy-related myelodysplasia and acute myeloid leukemia. Leukemia 17:1813–1819.
Christiansen DH, Andersen MK, Pedersen-Bjergaard J (2004) Mutations of AML1 are common in therapy-related myelodysplasia following therapy with alkylating agents and are significantly associated with deletion or loss of chromosome arm 7q and with subsequent leukemic transformation. Blood 104:1474–1481
Jäger R, Gisslinger H, Passamonti F, Rumi E, Berg T, Gisslinger B, Pietra D, Harutyunyan A, Klampfl T, Olcaydu D, Cazzola M, Kralovics R (2010) Deletions of the transcription factor ikaros in myeloproliferative neoplasms. Leukemia 24:1290–1298
Hussein K (2012) Pathobiology of the microRNA system. Pathologe 33:70–78
Rhyasen GW, Starczynowski DT (2012) Deregulation of microRNAs in myelodysplastic syndrome. Leukemia 26:13–22
Zhan H, Cardozo C, Raza A (2013) MicroRNAs in myeloproliferative neoplasms. Br J Haematol 161:471–483
Venturini L, Battmer K, Castoldi M, Schultheis B, Hochhaus A, Muckenthaler MU, Ganser A, Eder M, Scherr M (2007) Expression of the miR-17-92 polycistron in chronic myeloid leukemia (CML) CD34+ cells. Blood 109:4399–4405
Hussein K, Theophile K, Dralle W, Wiese B, Kreipe H, Bock O (2009) MicroRNA expression profiling of megakaryocytes in primary myelofibrosis and essential thrombocythemia. Platelets 20:391–400
Norfo R, Zini R, Pennucci V, Bianchi E, Salati S, Guglielmelli P, Bogani C, Fanelli T, Mannarelli C, Rosti V, Pietra D, Salmoiraghi S, Bisognin A, Ruberti S, Rontauroli S, Sacchi G, Prudente Z, Barosi G, Cazzola M, Rambaldi A, Bortoluzzi S, Ferrari S, Tagliafico E, Vannucchi AM, Manfredini R; Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative Investigators (2014) MiRNA-mRNA integrative analysis in primary myelofibrosis CD34+ cells: role of miR-155/JARID2 axis in abnormal megakaryopoiesis. Blood 124:e21–e32
Gebauer N, Bernard V, Gebauer W, Feller AC, Merz H (2013) MicroRNA expression and JAK2 allele burden in bone marrow trephine biopsies of polycythemia vera, essential thrombocythemia and early primary myelofibrosis. Acta Haematol 129:251–256
Lopotová T, Záčková M, Klamová H, Moravcová J (2011) MicroRNA-451 in chronic myeloid leukemia: miR-451-BCR-ABL regulatory loop? Leuk Res 35:974–977
Albano F, Anelli L, Zagaria A, Coccaro N, Casieri P, Minervini A, Specchia G (2012) SETBP1 and miR_4319 dysregulation in primary myelofibrosis progression to acute myeloid leukemia. J Hematol Oncol 5:48
Starczynowski DT, Kuchenbauer F, Argiropoulos B, Sung S, Morin R, Muranyi A, Hirst M, Hogge D, Marra M, Wells RA, Buckstein R, Lam W, Humphries RK, Karsan A (2010) Identification of miR-145 and miR-146a as mediators of the 5q- syndrome phenotype. Nat Med 16:49–58
Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM (2002) Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 99:15524–15529
Ruiz-Ballesteros E, Mollejo M, Mateo M, Algara P, Martínez P, Piris MA (2007) MicroRNA losses in the frequently deleted region of 7q in SMZL. Leukemia 21:2547–2259
Hussein K, Bock O, Theophile K, von Neuhoff N, Buhr T, Schlué J, Büsche G, Kreipe H (2009) JAK2(V617F) allele burden discriminates essential thrombocythemia from a subset of prefibrotic-stage primary myelofibrosis. Exp Hematol 37:1186–1193
Hussein K, Theophile K, Büsche G, Schlegelberger B, Göhring G, Kreipe H, Bock O (2010) Significant inverse correlation of microRNA-150/MYB and microRNA-222/p27 in myelodysplastic Syndrome. Leuk Res 34:328–334
Hussein K, Theophile K, Büsche G, Schlegelberger B, Göhring G, Kreipe H, Bock O (2010) Aberrant microRNA expression pattern in myelodysplastic bone marrow cells. Leuk Res 34:1169–1174
Li Y, Wang Y, Yu L, Sun C, Cheng D, Yu S, Wang Q, Yan Y, Kang C, Jin S, An T, Shi C, Xu J, Wei C, Liu J, Sun J, Wen Y, Zhao S, Kong Y (2013) miR-146b-5p inhibits glioma migration and invasion by targeting MMP16. Cancer Lett 339:260–269
Wang J, Wang Y, Han J, Li Y, Xie C, Xie L, Shi J, Zhang J, Yang B, Chen D, Meng X (2015) Integrated analysis of microRNA and mRNA expression profiles in the left atrium of patients with nonvalvular paroxysmal atrial fibrillation: role of miR-146b-5p in atrial fibrosis. Heart Rhythm 12:1018–1026
Boultwood J, Pellagatti A, Cattan H, Lawrie CH, Giagounidis A, Malcovati L, Della Porta MG, Jädersten M, Killick S, Fidler C, Cazzola M, Hellström-Lindberg E, Wainscoat JS (2007) Gene expression profiling of CD34+ cells in patients with the 5q- syndrome. Br J Haematol 139:578–589
Chen G, Zeng W, Miyazato A, Billings E, Maciejewski JP, Kajigaya S, Sloand EM, Young NS (2004) Distinctive gene expression profiles of CD34 cells from patients with myelodysplastic syndrome characterized by specific chromosomal abnormalities. Blood 104:4210–4218
Krejčík Z, Beličková M, Hruštincová A, Kléma J, Zemanová Z, Michalová K, Čermák J, Jonášová A, Dostálová MM (2015) Aberrant expression of the microRNA cluster in 14q32 is associated with del(5q) myelodysplastic syndrome and lenalidomide treatment. Cancer Genet 208:156–161
Ha JS, Jung HR (2015) Up-regulation of MicroRNA 146b is associated with myelofibrosis in myeloproliferative neoplasms. Ann Clin Lab Sci 45:308–314
Guglielmelli P, Tozzi L, Pancrazzi A, Bogani C, Antonioli E, Ponziani V, Poli G, Zini R, Ferrari S, Manfredini R, Bosi A, Vannucchi AM; MPD Research Consortium (2007) MicroRNA expression profile in granulocytes from primary myelofibrosis patients. Exp Hematol 35:1708–1718
Geraldo MV, Yamashita AS, Kimura ET (2013) MicroRNA miR-146b-5p regulates signal transduction of TGF-β by repressing SMAD4 in thyroid cancer. Oncogene 31:1910–1922
Bock O, Muth M, Theophile K, Winter M, Hussein K, Büsche G, Kröger N, Kreipe H (2009) Identification of new target molecules PTK2, TGFBR2 and CD9 overexpressed during advanced bone marrow remodelling in primary myelofibrosis. Br J Haematol 146:510–520
Compliance with ethical standards
All authors confirm compliance with ethical standards.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
None.
Conflict of interest statement
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 134 kb)
Rights and permissions
About this article
Cite this article
Stucki-Koch, A., Hauck, G., Kreipe, H. et al. MicroRNA expression profiles in BCR-ABL-negative primary myelofibrosis with chromosome 7q defects. J Hematopathol 8, 203–208 (2015). https://doi.org/10.1007/s12308-015-0258-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-015-0258-z