Abstract
Although several epidemiological studies assessed the relationship between thyrotropin and risk of Alzheimer’s disease in the elderly, the results were inconsistent. A systematic review and meta-analysis of cohort studies was conducted to assess the impact of serum thyrotropin levels on Alzheimer’s disease risk. PubMed, Embase, and Web of Science were searched through September 20, 2014 to identify cohort studies on the relationship between serum thyrotropin levels and risk of Alzheimer’s disease in the elderly. Pooled relative risks (RR) and 95 % confidence intervals (95 % CI) were calculated to assess the risk of Alzheimer’s disease according to serum thyrotropin levels. Eight prospective cohort studies were included, with a total of 9456 participants and 640 cases of Alzheimer’s disease. Low thyrotropin level was significantly associated with an increased risk of Alzheimer’s disease (fixed RR = 1.69, 95 % CI 1.31–2.19, P < 0.001; I 2 = 38.0 %). High thyrotropin level was also significantly associated with an increased risk of Alzheimer’s disease (fixed RR = 1.70, 95 % CI 1.18–2.45, P = 0.005; I 2 = 42.2 %) when compared with normal thyrotropin level. When using random effect model, low thyrotropin level was still significantly associated with risk of Alzheimer’s disease (random RR = 1.65, 95 % CI 1.14–2.37, P = 0.007), but high thyrotropin level was not (random RR = 1.54, 95 % CI 0.88–2.68, P = 0.129). When investigating thyrotropin levels continuously, an inverse but not significant association between serum thyrotropin levels and Alzheimer’s disease risk was observed (per standard deviation increment of thyrotropin: RR = 0.89, 95 % CI 0.78–1.01, P = 0.06; I 2 = 31.3 %). This meta-analysis supports that low thyrotropin level is significantly associated with an increased risk of Alzheimer’s disease in the elderly.
Similar content being viewed by others
Introduction
There are about 33.9 million people suffering from Alzheimer’s disease worldwide, and the prevalence is expected to increase obviously in the next 40 years [1, 2]. Previous studies have suggested that the disease has multiple causes and has a complex pathogenesis [1, 3]. Recent researches in molecular and cell biology, genetics, and biochemistry have revealed some molecular mechanisms of Alzheimer’s disease [4–7]. Despite intensive investment of Alzheimer’s disease and treatments aiming to alleviate symptoms in the past two decades, there is lack of effective treatment for Alzheimer’s disease [3, 8, 9]. To enable the development of effective prevention and disease-modifying treatment for Alzheimer’s disease, a better understanding of its pathogenesis is urgently needed. Identification of potential risk and protective factors for Alzheimer’s disease and cognitive impairment can help us get a better understanding of the pathogenesis and to establish effective interventions to reduce Alzheimer’s disease risk [10]. There are a number of modifiable risk factors identified for Alzheimer’s disease, such as diabetes, obesity, smoking, and physical inactivity [2, 10]. Thyroid hormones are important regulators in the development of fetal central nervous system and play important roles in the cognitive function after development [11–13]. Clinical hypothyroidism has long been recognized as a potential cause of cognitive impairment and Alzheimer’s disease [14–16]. Several studies also reported that serum thyrotropin (thyroid-stimulating hormone; TSH) levels were associated with cognitive function and Alzheimer’s disease [17–19]. However, some other investigations did not find the association between serum thyrotropin levels and Alzheimer’s disease [20–22]. Thus, previous epidemiological studies assessing the relationship between thyrotropin and risk of Alzheimer’s disease reported inconsistent findings. To get a comprehensive assessment of the impact of serum thyrotropin levels on Alzheimer’s disease risk, a systematic review and meta-analysis of cohort studies was conducted. This meta-analysis was performed according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement [23].
Methods
Search Strategy
PubMed, Embase, and Web of Science were searched to identify cohort studies on the relationship between serum thyrotropin levels and risk of Alzheimer’s disease. Unpublished data were mainly searched by searching in Google Scholar. Terms searched were as follows: (dementia OR Alzheimer’s Disease OR Alzheimer Disease OR cognitive decline OR cognitive dysfunction OR cognitive impairment or cognition disorders) AND (thyroid stimulating hormone OR thyrotropin OR tsh or thyroid disorders OR thyroid dysfunction OR thyroid function OR thyroid hormones) AND (cohort OR follow up OR prospective OR risk OR nested case-control OR risk ratio OR relative risk OR longitudinal). There was no language in the literature search. The last search was performed on September 20, 2014. The reference lists of relevant reviews or studies were also retrieved to find other potentially relevant studies.
Study Selection
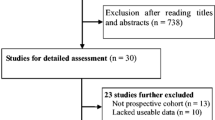
Studies were selected by two investigators independently using the following inclusion criteria: (1) using a prospective or retrospective design, or nested case-control studies; (2) having clearly stated diagnostic criteria for Alzheimer’s disease or dementia; (3) having data of baseline thyrotropin levels; (4) estimates of the association between serum thyrotropin levels and risk of Alzheimer’s disease; (5) follow-up was more than 2 years; and (6) relative risk (RR) estimate, hazard ratio (HR), or odds ratio (OR) with 95 % confidence intervals (95 % CIs) were reported. If multiple reports used the same population for calculating association measures, we only included the analysis based on the largest number of participants. We excluded studies that were cross-sectional, or case-control studies, studies that examined exposure to other factors but not thyrotropin levels, studies in which the outcome was vascular dementia but not dementia or Alzheimer’s disease, and studies without risk estimates of Alzheimer’s disease according to baseline thyrotropin levels. If two studies used the same study population during the same time period, we included only the study with larger sample size and longer follow-up (Fig. 1).
Data Extraction and Quality Assessment
Two investigators performed the data extraction independently. Discussion was undertaken post-extraction by the investigators to resolve any differences of opinion, and a third investigator was consulted when agreement could not be reached. From each included study, the following information was extracted: first author’s name, study design, age of participants, gender distribution, country, duration of follow-up, number of cases and non-cases, diagnostic criteria for Alzheimer’s disease or dementia, confounding factors, risk estimates, and corresponding 95 % confidence intervals for each category of baseline thyrotropin levels. For studies which reported risk estimates with multiple-level adjustment, we extracted risk estimates with the most adjustment. Quality assessment was conducted according to the Newcastle-Ottawa criteria for cohort studies [24]. A maximum of 9 points was assigned to each study: 4 for selection, 2 for comparability, and 3 for assessment of outcomes. The scores of 0–3, 4–6, and 7–9 were considered as low, moderate, and high quality, respectively.
Statistical Analysis
In the meta-analysis, RRs were used for risk estimates, and HRs and ORs used in some included studies were also treated as RRs directly. As different studies used different assessment methods and reported different exposure categories of baseline thyrotropin levels, we firstly pooled the study-specific risk estimate for low category of thyrotropin levels compared with the middle category of thyrotropin levels using meta-analysis. The study-specific risk estimate for high category of thyrotropin levels compared with the middle category of thyrotropin levels was also pooled for the meta-analysis. For studies which investigated thyrotropin levels continuously, risk estimate per standard deviation (SD) increment in thyrotropin levels was further pooled using meta-analysis. In the meta-analysis, between-study heterogeneity was estimated using I 2 statistic method, and the values of 25, 50, and 75 % represented low, moderate, and high degrees of heterogeneity [25]. When there were moderate or high degrees of heterogeneity, random effects model of DerSimonian and Laird was used to pool data, which took variation both within and between studies into consideration [26]. Otherwise, when there was no obvious heterogeneity, the fixed effect model of Mantel-Haenszel method was used to calculate the pooled effect estimates [27]. Sensitivity analysis was performed by omitting one study by turns and examining the influence of each individual study on the pooled risk estimates. Sensitivity analysis was also conducted by comparing the pooled outcomes from fixed effect model with that from random effects model. Publication bias was estimated by visual inspection of a funnel plot, and asymmetric funnel plot indicated possible risk of publication bias. In addition, Egger’s linear regression test (significant at P < 0.1) was also used to assess publication bias [28]. For the existence of publication bias, the trim and fill method was used to correct the asymmetry of funnel plot from publication bias and provide adjusted estimates by adding several estimated missing studies [29]. All analyses were conducted using STATA 12.0 (StataCorp, College Station, TX, USA). A two-sided P value less than 0.05 was considered as statistically significant.
Results
Study Selection and Characteristics
A total of 957 abstracts were identified through literature search, but only 21 studies were preliminarily considered eligible and were further assessed for eligibility by reviewing full-text [14, 17–22, 30–43]. After reviewing full-text, 13 articles were excluded because of not being prospective cohort studies or lack of usable data [14, 18, 30–40]. Eight prospective cohort studies were finally included into the meta-analysis [17, 19–22, 41–43]. Those 8 studies involved a total of 9456 participants and 640 cases of Alzheimer’s disease [17, 19–22, 41–43]. The main characteristics of those eight included studies are shown in Table 1. The mean time of follow-up ranged from 2 to 12.7 years. As to the quality, the scores of included studies ranged from 5 to 8, and the average score for the quality assessment was 6.7 [17, 19–22, 41–43]. Thus, all included studies had moderate or high quality [17, 19–22, 41–43]. As shown in Table 1, those studies used different assessment methods and reported different exposure categories of baseline thyrotropin levels (Table 1). One article reported data by gender and thus was treated as two individual studies [19]. Among all included studies, most studies are from Caucasian populations, but only one study was from Asian population [42]. There were five studies [19, 20, 41, 42] reporting risk estimate for low category of thyrotropin levels compared with the middle category of thyrotropin levels, three studies [19, 20] reporting risk estimate for high category of thyrotropin levels compared with the middle category of thyrotropin levels, and six studies [17, 20–22, 41, 43] investigating thyrotropin levels continuously and reporting risk estimate per SD increment in thyrotropin levels.
Thyrotropin and Alzheimer’s Disease Risk
There was no obvious between-study heterogeneity in those studies [19, 20, 41, 42] reporting risk estimate for low category of thyrotropin levels compared with the middle category of thyrotropin levels (I 2 = 38.0 %). Meta-analysis using fixed effect model showed that low thyrotropin level was significantly associated with an increased risk of Alzheimer’s disease (fixed RR = 1.69, 95 % CI 1.31–2.19, P < 0.001) (Fig. 2). Sensitivity analysis by omitting one study by turns showed that none of those included studies had obvious influence on the pooled risk estimates, which showed the stability of pooled in this meta-analysis. When using random effect model, low thyrotropin level was still significantly associated with risk of Alzheimer’s disease (random RR = 1.65, 95 % CI 1.14–2.37, P = 0.007). Subgroup analysis by gender showed that low thyrotropin level was still significantly associated with risk of Alzheimer’s disease in women (RR = 2.20, 95 % CI 1.34–3.61).
There was no obvious between-study heterogeneity in those studies [19, 20] reporting risk estimate for high category of thyrotropin levels compared with the middle category of thyrotropin levels (I 2 = 42.2 %). Meta-analysis using fixed effect model showed that high thyrotropin level was significantly associated with an increased risk of Alzheimer’s disease (fixed RR = 1.70, 95 % CI 1.18–2.45, P = 0.005) when compared with normal thyrotropin level (Fig. 3). When using random effect model, high thyrotropin level was not associated with risk of Alzheimer’s disease (random RR = 1.54, 95 % CI 0.88–2.68, P = 0.129). Sensitivity analysis by omitting one study by turns showed that several studies had obvious influence on the pooled risk estimates, which showed the pooled results in this meta-analysis were not stable. Subgroup analysis by gender showed that high thyrotropin level was still significantly associated with risk of Alzheimer’s disease in women (RR = 2.20, 95 % CI 1.39–3.48).
There was no obvious between-study heterogeneity in those six studies [17, 20–22, 41, 43] investigating thyrotropin levels continuously and reporting risk estimate per SD increment in thyrotropin levels (I 2 = 31.3 %). When investigating thyrotropin levels continuously, an inverse but not significant association between serum thyrotropin levels and risk of Alzheimer’s disease was observed (per standard deviation increment of thyrotropin: fixed RR = 0.89, 95 % CI 0.78–1.01, P = 0.06; random RR = 0.87, 95 % CI 0.74–1.03, P = 0.11) (Fig. 4). Sensitivity analysis by omitting one study by turns showed that several studies had obvious influence on the pooled risk estimates, which showed the pooled results in this meta-analysis were not stable. After excluding the study from Asian countries, there was still no obvious association between serum thyrotropin levels and risk of Alzheimer’s disease in Caucasians.
Publication Bias
Publication bias was firstly estimated by visual inspection of funnel plots. The funnel plot for meta-analysis relating low thyrotropin level did not suggest publication bias (Fig. 5), but the funnel plot for the meta-analysis investigating thyrotropin levels continuously suggested possible risk of publication bias. Egger’s test further showed that there was no obvious risk of publication bias in the meta-analysis relating low thyrotropin level (P = 0.74), but there was obvious risk of publication bias in the meta-analysis investigating thyrotropin levels continuously (P = 0.01). When using trim and fill method, no estimated missing study was added, and there was no change in the pooled risk estimates.
Discussion
This meta-analysis aimed to get a comprehensive assessment on the relationship between serum thyrotropin levels and risk of Alzheimer’s disease in the elderly. It is the first meta-analysis of thyrotropin with Alzheimer’s disease risk and provides a more precise evaluation on the impact of thyrotropin on Alzheimer’s disease risk than single study. Eight prospective cohort studies were included, with a total of 9456 participants and 640 cases of Alzheimer’s disease [17, 19–22, 41–43]. The findings support that low thyrotropin level was significantly associated with an increased risk of Alzheimer’s disease compared with the middle category of thyrotropin levels (Fig. 1). Sensitivity analyses further proved the stability of pooled risk estimates. Thus, our meta-analysis provides a strong evidence for the relationship between low serum thyrotropin levels and increased risk of Alzheimer’s disease.
The relationship between high serum thyrotropin levels and Alzheimer’s disease risk is still not well established. Though an obvious association was found in the meta-analysis using fixed effect model, we failed to identify a significant association between high serum thyrotropin levels and Alzheimer’s disease risk when using random effect model. As shown in Fig. 3, there were only three studies available now, which could result in a lower statistical power. In addition, those included studies used different exposure categories of baseline thyrotropin levels to assess risk of Alzheimer’s disease, which could result in high degrees of between-study heterogeneity (I 2 = 42.2 %) and decrease the credibility of the pooled risk estimates. Thus, more well-designed prospective studies are needed to further assess the relationship between high serum thyrotropin levels and Alzheimer’s disease risk in the future.
It has been estimated that about 20 % of the total population will be elder adults (aged ≥60 years). With the aging population, there will be an estimated 100 million people suffering from Alzheimer’s disease worldwide in 2050 [2]. Thus, it is urgent for us to find some modifiable risk factors and establish some effective interventions to reduce Alzheimer’s disease risk [2]. The findings in our meta-analysis have some important clinical implications. Our meta-analysis provides a strong evidence for the relationship between low serum thyrotropin levels and increased risk of Alzheimer’s disease and individuals with low serum thyrotropin levels are at high risk of developing Alzheimer’s disease. Therefore, elderly subjects with lower serum thyrotropin levels might require more intensive interventions to reduce the risk of cognitive impairment and Alzheimer’s disease. In addition, the obvious relationship between thyrotropin and Alzheimer’s disease also indicates that thyrotropin may be involved in the development of Alzheimer’s disease. However, the pathogenesis of Alzheimer’s disease is still not well known and the mechanism underlying the association above is also still unclear [4, 6, 9, 44]. Currently, there are several possible explanations. The neurodegeneration in the brain causing Alzheimer’s disease may also reduce the secretion of thyrotropin-releasing hormone. Besides the hypothalamus, other brain areas also contain thyrotropin-releasing hormone secreting neurons, such as the C1–3 adrenergic neurons of the brainstem [45]. Thyrotropin-releasing hormone plays a generalized role as a central nervous system neurotransmitter, and the neurodegeneration in Alzheimer’s disease may lead to the disturbance of thyrotropin-releasing hormone [46, 47], reducing serum thyrotropin successively. Another explanation is that the low thyrotropin reflects true thyroid overactivity and thyroid hormone excess, which causes toxic effect to the brain. More researches are needed to explore possible explanations for the role of thyrotropin in the development of Alzheimer’s disease.
There were several limitations to be taken into consideration. Firstly, the main limitation was the lack of individual participant data from included studies. As shown in Table 1, different studies used different assessment methods and reported different exposure categories of baseline thyrotropin levels. In addition, confounding factors used in the adjusted analyses were also different between those included studies. A meta-analysis of individual participant data can decrease the influence of heterogeneity among those studies and can provide a more in-depth analysis on the role of thyrotropin in the development of Alzheimer’s disease [48]. Secondly, another important factor that could modify the effect of baseline thyrotropin levels on Alzheimer’s disease risk was changes of thyrotropin levels during the follow-up. None of those included studies provided the estimates adjusted for changes of thyrotropin levels during follow-up. Thus, currently available studies were unable to exclude the possible influence of changes of thyrotropin levels on the association between baseline thyrotropin levels and Alzheimer’s disease risk. Thirdly, there was still limited number of eligible studies on the relationship between thyrotropin and Alzheimer’s disease. In the meta-analysis of the association between high serum thyrotropin levels and Alzheimer’s disease risk, there were only three studies, which could increase risk of random error and increase risk of bias. In addition, there was only one study in the subgroup analyses by gender, which might reduce the credibility of results in the subgroup analyses. Thus, more studies are needed to further assess the relationship between serum thyrotropin levels and Alzheimer’s disease risk in the future. Finally, the findings in the meta-analysis may not be generalized to all ethnic populations. As shown in Table 1, most studies were performed in Europe or USA, but only one study was performed in Asians. The association between serum thyrotropin levels and Alzheimer’s disease risk in Asians or Africans is still not well investigated and needs future studies.
In summary, this meta-analysis supports that low thyrotropin level is significantly associated with an increased risk of Alzheimer’s disease in the elderly. More well-designed prospective cohort studies are needed to further identify the association between serum thyrotropin levels and Alzheimer’s disease risk.
References
Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E (2011) Alzheimer’s disease. Lancet 377:1019–1031
Barnes DE, Yaffe K (2011) The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 10:819–828
Huang Y, Mucke L (2012) Alzheimer mechanisms and therapeutic strategies. Cell 148:1204–1222
Lu JX, Qiang W, Yau WM, Schwieters CD, Meredith SC, Tycko R (2013) Molecular structure of beta-amyloid fibrils in Alzheimer’s disease brain tissue. Cell 154:1257–1268
Reitz C, Jun G, Naj A et al (2013) Variants in the ATP-binding cassette transporter (ABCA7), apolipoprotein E 4, and the risk of late-onset Alzheimer disease in African Americans. JAMA 309:1483–1492
Guerreiro R, Wojtas A, Bras J et al (2013) TREM2 variants in Alzheimer’s disease. N Engl J Med 368:117–127
Liu CC, Kanekiyo T, Xu H, Bu G (2013) Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 9:106–118
Langbaum JB, Fleisher AS, Chen K et al (2013) Ushering in the study and treatment of preclinical Alzheimer disease. Nat Rev Neurol 9:371–381
Jack CR Jr, Knopman DS, Jagust WJ et al (2013) Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12:207–216
Selkoe DJ (2012) Preventing Alzheimer’s disease. Science (New York, NY) 337:1488–1492
Thompson CC, Weinberger C, Lebo R, Evans RM (1987) Identification of a novel thyroid hormone receptor expressed in the mammalian central nervous system. Science (New York, NY) 237:1610–1614
Thorpe-Beeston JG, Nicolaides KH, Felton CV, Butler J, McGregor AM (1991) Maturation of the secretion of thyroid hormone and thyroid-stimulating hormone in the fetus. N Engl J Med 324:532–536
Laurberg P (2009) Thyroid function: thyroid hormones, iodine and the brain-an important concern. Nat Rev Endocrinol 5:475–476
Parsaik AK, Singh B, Roberts RO et al (2014) Hypothyroidism and risk of mild cognitive impairment in elderly persons: a population-based study. JAMA Neurol 71:201–207
Suhanov AV, Pilipenko PI, Korczyn AD et al (2006) Risk factors for Alzheimer’s disease in Russia: a case-control study. Eur J Neurol Off J Eur Fed Neurol Soc 13:990–995
Breteler MM, van Duijn CM, Chandra V et al (1991) Medical history and the risk of Alzheimer’s disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol 20(Suppl 2):S36–S42
Annerbo S, Wahlund LO, Lokk J (2006) The significance of thyroid-stimulating hormone and homocysteine in the development of Alzheimer’s disease in mild cognitive impairment: a 6-year follow-up study. Am J Alzheim Dis Other Dementias 21:182–188
Hogervorst E, Huppert F, Matthews FE, Brayne C (2008) Thyroid function and cognitive decline in the MRC Cognitive Function and Ageing Study. Psychoneuroendocrinology 33:1013–1022
Tan ZS, Beiser A, Vasan RS et al (2008) Thyroid function and the risk of Alzheimer disease: the Framingham Study. Arch Intern Med 168:1514–1520
de Jong FJ, den Heijer T, Visser TJ et al (2006) Thyroid hormones, dementia, and atrophy of the medial temporal lobe. J Clin Endocrinol Metab 91:2569–2573
Annerbo S, Kivipelto M, Lokk J (2009) A prospective study on the development of Alzheimer’s disease with regard to thyroid-stimulating hormone and homocysteine. Dement Geriatr Cogn Disord 28:275–280
de Jong FJ, Masaki K, Chen H et al (2009) Thyroid function, the risk of dementia and neuropathologic changes: the Honolulu-Asia aging study. Neurobiol Aging 30:600–606
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269
Wells G, Shea B, O’connell D, et al. (2000) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm)
Higgins J, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22:719–748
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56:455–463
Beydoun MA, Beydoun HA, Shroff MR, Kitner-Triolo MH, Zonderman AB (2012) Serum leptin, thyroxine and thyroid-stimulating hormone levels interact to affect cognitive function among US adults: evidence from a large representative survey. Neurobiol Aging 33:1730–1743
de Jongh RT, Lips P, van Schoor NM et al (2011) Endogenous subclinical thyroid disorders, physical and cognitive function, depression, and mortality in older individuals. Eur J Endocrinol Eur Fed Endocr Soc 165:545–554
Formiga F, Ferrer A, Padros G, Contra A, Corbella X, Pujol R (2014) Thyroid status and functional and cognitive status at baseline and survival after 3 years of follow-up: the OCTABAIX study. Eur J Endocrinol Eur Fed Endocr Soc 170:69–75
Forti P, Olivelli V, Rietti E et al (2012) Serum thyroid-stimulating hormone as a predictor of cognitive impairment in an elderly cohort. Gerontology 58:41–49
Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frolich M, Westendorp RG (2004) Thyroid status, disability and cognitive function, and survival in old age. JAMA 292:2591–2599
Regal PJ (2012) Antithyroid antibodies, cognition and instrumental activities of daily living in the elderly. Int J Geriatr Psychiatr 27:1317–1318
Vadiveloo T, Donnan PT, Cochrane L, Leese GP (2011) The Thyroid Epidemiology, Audit, and Research Study (TEARS): morbidity in patients with endogenous subclinical hyperthyroidism. J Clin Endocrinol Metab 96:1344–1351
Volpato S, Guralnik JM, Fried LP, Remaley AT, Cappola AR, Launer LJ (2002) Serum thyroxine level and cognitive decline in euthyroid older women. Neurology 58:1055–1061
Wahlin A, Bunce D, Wahlin TB (2005) Longitudinal evidence of the impact of normal thyroid stimulating hormone variations on cognitive functioning in very old age. Psychoneuroendocrinology 30:625–637
Wijsman LW, de Craen AJ, Trompet S et al (2013) Subclinical thyroid dysfunction and cognitive decline in old age. PLoS One 8:e59199
Yamamoto N, Ishizawa K, Ishikawa M et al (2012) Cognitive function with subclinical hypothyroidism in elderly people without dementia: one year follow up. Geriatr Gerontol Int 12:164–165
Kalmijn S, Mehta KM, Pols HA, Hofman A, Drexhage HA, Breteler MM (2000) Subclinical hyperthyroidism and the risk of dementia. The Rotterdam study. Clin Endocrinol 53:733–737
Moon JH, Park YJ, Kim TH et al (2014) Lower-but-normal serum TSH level is associated with the development or progression of cognitive impairment in elderly: Korean Longitudinal Study on Health and Aging (KLoSHA). J Clin Endocrinol Metab 99:424–432
Yeap BB, Alfonso H, Chubb SA et al (2012) Higher free thyroxine levels predict increased incidence of dementia in older men: the Health in Men Study. J Clin Endocrinol Metab 97:E2230–E2237
Rhinn H, Fujita R, Qiang L, Cheng R, Lee JH, Abeliovich A (2013) Integrative genomics identifies APOE epsilon4 effectors in Alzheimer’s disease. Nature 500:45–50
Liposits Z, Paull WK, Wu P, Jackson IM, Lechan RM (1987) Hypophysiotrophic thyrotropin releasing hormone (TRH) synthesizing neurons. Ultrastructure, adrenergic innervation and putative transmitter action. Histochemistry 88:1–10
Gan EH, Pearce SH (2012) Clinical review: the thyroid in mind: cognitive function and low thyrotropin in older people. J Clin Endocrinol Metab 97:3438–3449
Annerbo S, Lokk J (2013) A clinical review of the association of thyroid stimulating hormone and cognitive impairment. ISRN Endocrinol 2013:856017
Simmonds MC, Higgins JP, Stewart LA, Tierney JF, Clarke MJ, Thompson SG (2005) Meta-analysis of individual patient data from randomized trials: a review of methods used in practice. Clin Trials (London, England) 2:209–217
Conflicts of Interest
None declared.
Funding
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yunyang Wang, Qi Sheng, and Xu Hou contributed equally to the study.
Rights and permissions
About this article
Cite this article
Wang, Y., Sheng, Q., Hou, X. et al. Thyrotropin and Alzheimer’s Disease Risk in the Elderly: a Systematic Review and Meta-Analysis. Mol Neurobiol 53, 1229–1236 (2016). https://doi.org/10.1007/s12035-014-9078-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-9078-x