Abstract
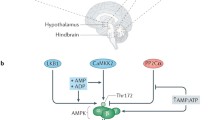
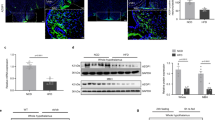
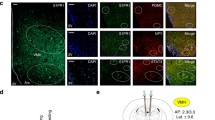
PAS kinase (PASK) is a nutrient sensor that is highly conserved throughout evolution. PASK-deficient mice reveal a metabolic phenotype similar to that described in S6 kinase-1 S6K1-deficient mice that are protected against obesity. Hypothalamic metabolic sensors, such as AMP-activated protein kinase (AMPK) and the mammalian target of rapamycin (mTOR), play an important role in feeding behavior, the homeostasis of body weight, and energy balance. These sensors respond to changes in nutrient levels in the hypothalamic areas involved in feeding behavior and in neuroblastoma N2A cells, and we have recently reported that those effects are modulated by the anorexigenic peptide glucagon-like peptide-1 (GLP-1). Here, we identified PASK in both N2A cells and rat VMH and LH areas and found that its expression is regulated by glucose and GLP-1. High levels of glucose decreased Pask gene expression. Furthermore, PASK-silenced N2A cells record an impaired response by the AMPK and mTOR/S6K1 pathways to changes in glucose levels. Likewise, GLP-1 effect on the activity of AMPK, S6K1, and other intermediaries of both pathways and the regulatory role at the level of gene expression were also blocked in PASK-silenced cells. The absence of response to low glucose concentrations in PASK-silenced cells correlates with increased ATP content, low expression of mRNA coding for AMPK upstream kinase LKB1, and enhanced activation of S6K1. Our findings indicate that, at least in N2A cells, PASK is a key kinase in GLP-1 actions and exerts a coordinated response with the other metabolic sensors, suggesting that PASK might play an important role in feeding behavior.









Similar content being viewed by others
References
Grose JH, Smith TL, Sabic H, Rutter J (2007) Yeast PAS kinase coordinates glucose partitioning in response to metabolic and cell integrity signaling. EMBO J 26(23):4824–4830
Smith TL, Rutter J (2007) Regulation of glucose partitioning by PAS kinase and Ugp1 phosphorylation. Mol Cell 26(4):491–499
Hao HX, Rutter J (2008) The role of PAS kinase in regulating energy metabolism. IUBMB Life 60(4):204–209
Hardie DG, Carling D, Carlson M (1998) The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem 67:821–855
Hardie DG, Ross FA, Hawley SA (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13(4):251–262
Rutter GA, Da Silva XG, Leclerc I (2003) Roles of 5′-AMP-activated protein kinase (AMPK) in mammalian glucose homoeostasis. Biochem J 375(Pt 1):1–16
Alessi DR, Pearce LR, Garcia-Martinez JM (2009) New insights into mTOR signaling: mTORC2 and beyond. Sci Signal 2(67):pe27
Foster KG, Fingar DC (2010) Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem 285(19):14071–14077
Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12(1):21–35
Proud CG (2002) Regulation of mammalian translation factors by nutrients. Eur J Biochem 269(22):5338–5349
Gingras AC, Raught B, Sonenberg N (2001) Regulation of translation initiation by FRAP/mTOR. Genes Dev 15(7):807–826
Inoki K, Zhu T, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115(5):577–590
Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30(2):214–226
da Silva XG, Rutter J, Rutter GA (2004) Involvement of Per-Arnt-Sim (PAS) kinase in the stimulation of preproinsulin and pancreatic duodenum homeobox 1 gene expression by glucose. Proc Natl Acad Sci U S A 101(22):8319–8324
Borter E, Niessen M, Zuellig R, Spinas GA, Spielmann P, Camenisch G, Wenger RH (2007) Glucose-stimulated insulin production in mice deficient for the PAS kinase PASKIN. Diabetes 56(1):113–117
Katschinski DM, Marti HH, Wagner KF, Shibata J, Eckhardt K, Martin F, Depping R, Paasch U, Gassmann M, Ledermann B, Desbaillets I, Wenger RH (2003) Targeted disruption of the mouse PAS domain serine/threonine kinase PASKIN. Mol Cell Biol 23(19):6780–6789
Hao HX, Cardon CM, Swiatek W, Cooksey RC, Smith TL, Wilde J, Boudina S, Abel ED, McClain DA, Rutter J (2007) PAS kinase is required for normal cellular energy balance. Proc Natl Acad Sci U S A 104(39):15466–15471
MacDonald PE, Rorsman P (2011) Per-arnt-sim (PAS) domain kinase (PASK) as a regulator of glucagon secretion. Diabetologia 54(4):719–721
Schlafli P, Borter E, Spielmann P, Wenger RH (2009) The PAS-domain kinase PASKIN: a new sensor in energy homeostasis. Cell Mol Life Sci 66(5):876–883
Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, Taylor KL, Trautmann ME, Kim DD, Kendall DM (2006) Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 8(4):436–447
Niswender K (2010) Diabetes and obesity: therapeutic targeting and risk reduction—a complex interplay. Diabetes Obes Metab 12(4):267–287
Hurtado-Carneiro V, Sanz C, Roncero I, Vazquez P, Blazquez E, Alvarez E (2012) Glucagon-like peptide 1 (GLP-1) can reverse AMP-activated protein kinase (AMPK) and S6 kinase (P70S6K) activities induced by fluctuations in glucose levels in hypothalamic areas involved in feeding behaviour. Mol Neurobiol 45(2):348–361
Leon Y, Sanz C, Giraldez F, Varela-Nieto I (1998) Induction of cell growth by insulin and insulin-like growth factor-I is associated with Jun expression in the otic vesicle. J Comp Neurol 398(3):323–332
Sanz C, Vazquez P, Blazquez C, Barrio PA, Alvarez Mdel M, Blazquez E (2010) Signaling and biological effects of glucagon-like peptide 1 on the differentiation of mesenchymal stem cells from human bone marrow. Am J Physiol Endocrinol Metab 298(3):E634–E643
Sanz C, Roncero I, Vazquez P, Navas MA, Blazquez E (2007) Effects of glucose and insulin on glucokinase activity in rat hypothalamus. J Endocrinol 193(2):259–267
Paxinos G, Watson C (2004) The brain in stereotaxic coordinates. Elseviere, New York
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162(1):156–159
Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, Klumperman J, Thorens B, Thomas G (2000) Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature 408(6815):994–997
Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G (2004) Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431(7005):200–205
Solomon A, De Fanti BA, Martinez JA (2006) Peripheral ghrelin participates in the glucostatic signaling mediated by the ventromedial and lateral hypothalamus neurons. Peptides 27(7):1607–1615
Lee K, Li B, Xi X, Suh Y, Martin RJ (2005) Role of neuronal energy status in the regulation of adenosine 5′-monophosphate-activated protein kinase, orexigenic neuropeptides expression, and feeding behavior. Endocrinology 146(1):3–10
Sanz C, Vazquez P, Navas MA, Alvarez E, Blazquez E (2008) Leptin but not neuropeptide Y up-regulated glucagon-like peptide 1 receptor expression in GT1-7 cells and rat hypothalamic slices. Metabolism 57(1):40–48
McCrimmon RJ, Fan X, Cheng H, McNay E, Chan O, Shaw M, Ding Y, Zhu W, Sherwin RS (2006) Activation of AMP-activated protein kinase within the ventromedial hypothalamus amplifies counterregulatory hormone responses in rats with defective counterregulation. Diabetes 55(6):1755–1760
Seo S, Ju S, Chung H, Lee D, Park S (2008) Acute effects of glucagon-like peptide-1 on hypothalamic neuropeptide and AMP activated kinase expression in fasted rats. Endocr J 55(5):867–874
Viollet B, Athea Y, Mounier R, Guigas B, Zarrinpashneh E, Horman S, Lantier L, Hebrard S, Devin-Leclerc J, Beauloye C, Foretz M, Andreelli F, Ventura-Clapier R, Bertrand L (2009) AMPK: lessons from transgenic and knockout animals. Front Biosci 14:19–44
Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, Alessi DR (2005) Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J 24(10):1810–1820
Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC (2005) The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310(5754):1642–1646
Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D (2007) Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J 403(1):139–148
Lopez M, Vidal-Puig A (2008) Brain lipogenesis and regulation of energy metabolism. Curr Opin Clin Nutr Metab Care 11(4):483–490
Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, Tschop MH, Shanabrough M, Cline G, Shulman GI, Coppola A, Gao XB, Horvath TL, Diano S (2008) UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature 454(7206):846–851
Kodiha M, Rassi JG, Brown CM, Stochaj U (2007) Localization of AMP kinase is regulated by stress, cell density, and signaling through the MEKERK1/2 pathway. Am J Physiol Cell Physiol 293(5):C1427–C1436
Chopra I, Li HF, Wang H, Webster KA (2012) Phosphorylation of the insulin receptor by AMP-activated protein kinase (AMPK) promotes ligand-independent activation of the insulin signalling pathway in rodent muscle. Diabetologia 55(3):783–794
Khamzina L, Veilleux A, Bergeron S, Marette A (2005) Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology 146(3):1473–1481
Al-Khouri AM, Ma Y, Togo SH, Williams S, Mustelin T (2005) Cooperative phosphorylation of the tumor suppressor phosphatase and tensin homologue (PTEN) by casein kinases and glycogen synthase kinase 3beta. J Biol Chem 280(42):35195–35202
Blanco-Aparicio C, Renner O, Leal JF, Carnero A (2007) PTEN, more than the AKT pathway. Carcinogenesis 28(7):1379–1386
Acknowledgments
This work was supported by grants from MICINN (SAF2006-0475 and SAF2009-11297), Ayudas del Programa de Creación y Consolidación de Grupos de Investigación UCM-Banco Santander (GR58/08, GR35/10A, GR35/10B and GR42/10), Fundación de Investigación Médica Mutua Madrileña and IODURE project, CIBER de Diabetes y Enfermedades Metabólicas Asociadas, an initiative of ISCIII (Ministerio de Ciencia e Innovación).
Author information
Authors and Affiliations
Corresponding author
Additional information
Elvira Alvarez and Carmen Sanz contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hurtado-Carneiro, V., Roncero, I., Blazquez, E. et al. PAS Kinase as a Nutrient Sensor in Neuroblastoma and Hypothalamic Cells Required for the Normal Expression and Activity of Other Cellular Nutrient and Energy Sensors. Mol Neurobiol 48, 904–920 (2013). https://doi.org/10.1007/s12035-013-8476-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8476-9