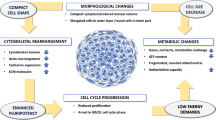
Abstract
Derivation of articular chondrocytes from human stem cells would advance our current understanding of chondrogenesis, and accelerate development of new stem cell therapies for cartilage repair. Chondrogenic differentiation of human embryonic stem cells (hESCs) has been studied using supplemental and cell-secreted morphogenetic factors. The use of bioreactors enabled insights into the effects of physical forces and controlled oxygen tension. In this study, we investigated the interactive effects of controlled variation of oxygen tension and chondrocyte-secreted morphogenetic factors on chondrogenic differentiation of hESCs in the embryoid body format (hESC-EB). Transient hypoxic culture (2 weeks at 5 % O2 followed by 1 week at 21 % O2) of hESC-EBs in medium conditioned with primary chondrocytes up-regulated the expression of SOX9 and suppressed pluripotent markers OCT4 and NANOG. Pellets derived from these cells showed significant up-regulation of chondrogenic genes (SOX9, COL2A1, ACAN) and enhanced production of cartilaginous matrix (collagen type II and proteoglycan) as compared to the pellets from hESC-EBs cultured under normoxic conditions. Gene expression profiles corresponded to those associated with native cartilage development, with early expression of N-cadherin (indicator of cell condensation) and late expression of aggrecan (ACAN, indicator of proteoglycan production). When implanted into highly vascularized subcutaneous area in immunocompromised mice for 4 weeks, pellets remained phenotypically stable and consisted of cartilaginous extracellular matrix (ECM), without evidence of dedifferentiation or teratoma formation. Based on these results, we propose that chondrogenesis in hESC can be synergistically enhanced by a control of oxygen tension and morphogenetic factors secreted by chondrocytes.







Similar content being viewed by others
References
Tuan, R. S., Boland, G., & Tuli, R. (2003). Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Research and Therapy, 5(1), 32–45.
Langer, R., & Vacanti, J. P. (1993). Tissue engineering. Science, 260(5110), 920–926.
Oldershaw, R. A. (2012). Cell sources for the regeneration of articular cartilage: the past, the horizon and the future. International Journal of Experimental Pathology, 93(6), 389–400.
Meinel, L., Hofmann, S., Karageorgiou, V., Zichner, L., Langer, R., Kaplan, D., et al. (2004). Engineering cartilage-like tissue using human mesenchymal stem cells and silk protein scaffolds. Biotechnology and Bioengineering, 88(3), 379–391.
Steck, E., Fischer, J., Lorenz, H., Gotterbarm, T., Jung, M., & Richter, W. (2009). Mesenchymal stem cell differentiation in an experimental cartilage defect: restriction of hypertrophy to bone-close neocartilage. Stem Cells and Development, 18(7), 969–978.
Estes, B. T., Diekman, B. O., Gimble, J. M., & Guilak, F. (2010). Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nature Protocols, 5(7), 1294–1311.
Stolzing, A., Jones, E., McGonagle, D., & Scutt, A. (2008). Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mechanisms of Ageing and Development, 129(3), 163–173.
Long, F., & Ornitz, D. M. (2013). Development of the endochondral skeleton. Cold Spring Harbor Perspectives in Biology, 5(1), a008334.
Pelttari, K., Winter, A., Steck, E., Goetzke, K., Hennig, T., Ochs, B. G., et al. (2006). Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis and Rheumatism, 54(10), 3254–3266.
Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., Waknitz, M. A., Swiergiel, J. J., Marshall, V. S., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science, 282(5391), 1145–1147.
Sa, S., & McCloskey, K. E. (2012). Stage-specific cardiomyocyte differentiation method for h7 and h9 human embryonic stem cells. Stem Cell Reviews, 8(4), 1120–1128.
Zhang, J., Wilson, G. F., Soerens, A. G., Koonce, C. H., Yu, J., Palecek, S. P., et al. (2009). Functional cardiomyocytes derived from human induced pluripotent stem cells. Circulation Research, 104(4), e30–41.
Marolt, D., Campos, I. M., Bhumiratana, S., Koren, A., Petridis, P., Zhang, G., et al. (2012). Engineering bone tissue from human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America, 109(22), 8705–8709.
Oldershaw, R. A., Baxter, M. A., Lowe, E. T., Bates, N., Grady, L. M., Soncin, F., et al. (2010). Directed differentiation of human embryonic stem cells toward chondrocytes. Nature Biotechnology, 28(11), 1187–1194.
Nakagawa, T., Lee, S. Y., & Reddi, A. H. (2009). Induction of chondrogenesis from human embryonic stem cells without embryoid body formation by bone morphogenetic protein 7 and transforming growth factor beta1. Arthritis and Rheumatism, 60(12), 3686–3692.
Toh, W. S., Lee, E. H., Guo, X. M., Chan, J. K., Yeow, C. H., Choo, A. B., et al. (2010). Cartilage repair using hyaluronan hydrogel-encapsulated human embryonic stem cell-derived chondrogenic cells. Biomaterials, 31(27), 6968–6980.
Amarilio, R., Viukov, S. V., Sharir, A., Eshkar-Oren, I., Johnson, R. S., & Zelzer, E. (2007). HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development, 134(21), 3917–3928.
Ezashi, T., Das, P., & Roberts, R. M. (2005). Low O2 tensions and the prevention of differentiation of hES cells. Proceedings of the National Academy of Sciences of the United States of America, 102(13), 4783–4788.
Koay, E. J., & Athanasiou, K. A. (2008). Hypoxic chondrogenic differentiation of human embryonic stem cells enhances cartilage protein synthesis and biomechanical functionality. Osteoarthritis and Cartilage, 16(12), 1450–1456.
Hwang, N. S., Varghese, S., & Elisseeff, J. (2008). Derivation of chondrogenically-committed cells from human embryonic cells for cartilage tissue regeneration. PLoS One, 3(6), e2498.
Hwang, N. S., Varghese, S., Lee, H. J., Zhang, Z., Ye, Z., Bae, J., et al. (2008). In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proceedings of the National Academy of Sciences of the United States of America, 105(52), 20641–20646.
Lima, E. G., Grace Chao, P. H., Ateshian, G. A., Bal, B. S., Cook, J. L., Vunjak-Novakovic, G., et al. (2008). The effect of devitalized trabecular bone on the formation of osteochondral tissue-engineered constructs. Biomaterials, 29(32), 4292–4299.
Yodmuang, S., Gadjanski, I., Chao, P. H., & Vunjak-Novakovic, G. (2013). Transient hypoxia improves matrix properties in tissue engineered cartilage. Journal of Orthopaedic Research, 31(4), 544–553.
Yang, L., Soonpaa, M. H., Adler, E. D., Roepke, T. K., Kattman, S. J., Kennedy, M., et al. (2008). Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature, 453(7194), 524–528.
Schmittgen, T. D., & Livak, K. J. (2008). Analyzing real-time PCR data by the comparative C(T) method. Nature Protocols, 3(6), 1101–1108.
Farndale, R. W., Buttle, D. J., & Barrett, A. J. (1986). Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochimica et Biophysica Acta, 883(2), 173–177.
Bruckner, P., Horler, I., Mendler, M., Houze, Y., Winterhalter, K. H., Eich-Bender, S. G., et al. (1989). Induction and prevention of chondrocyte hypertrophy in culture. Journal of Cell Biology, 109(5), 2537–2545.
Kisiday, J., Jin, M., Kurz, B., Hung, H., Semino, C., Zhang, S., et al. (2002). Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proceedings of the National Academy of Sciences of the United States of America, 99(15), 9996–10001.
Zhao, H., Ito, Y., Chappel, J., Andrews, N. W., Teitelbaum, S. L., & Ross, F. P. (2008). Synaptotagmin VII regulates bone remodeling by modulating osteoclast and osteblast secretion. Developmental Cell, 14(6), 914–925.
Tavella, S., Raffo, P., Tacchetti, C., Cancedda, R., & Castagnola, P. (1994). N-CAM and N-cadherin expression during in vitro chondrogenesis. Experimental Cell Research, 215(2), 354–362.
Meier, S., Solursh, M., & Vaerewyck, S. (1973). Modulation of extracellular matrix production by conditioned medium. American Zoologist, 13, 1051–1060.
Keller, G. M. (1995). In vitro differentiation of embryonic stem cells. Current Opinion in Cell Biology, 7(6), 862–869.
Kramer, J., Hegert, C., Guan, K., Wobus, A. M., Muller, P. K., & Rohwedel, J. (2000). Embryonic stem cell-derived chondrogenic differentiation in vitro: activation by BMP-2 and BMP-4. Mechanisms of Development, 92(2), 193–205.
Vats, A., Bielby, R. C., Tolley, N., Dickinson, S. C., Boccaccini, A. R., Hollander, A. P., et al. (2006). Chondrogenic differentiation of human embryonic stem cells: the effect of the micro-environment. Tissue Engineering, 12(6), 1687–1697.
Benjamin, S., Sheyn, D., Ben-David, S., Oh, A., Kallai, I., Li, N., et al. (2013). Oxygenated environment enhances both stem cell survival and osteogenic differentiation. Tissue Engineering Part A, 19(5–6), 748–758.
Muller, J., Benz, K., Ahlers, M., Gaissmaier, C., & Mollenhauer, J. (2011). Hypoxic conditions during expansion culture prime human mesenchymal stromal precursor cells for chondrogenic differentiation in three-dimensional cultures. Cell Transplantation, 20(10), 1589–1602.
Egli, R. J., Bastian, J. D., Ganz, R., Hofstetter, W., & Leunig, M. (2008). Hypoxic expansion promotes the chondrogenic potential of articular chondrocytes. Journal of Orthopaedic Research, 26(7), 977–985.
Meretoja, V. V., Dahlin, R. L., Wright, S., Kasper, F. K., & Mikos, A. G. (2013). The effect of hypoxia on the chondrogenic differentiation of co-cultured articular chondrocytes and mesenchymal stem cells in scaffolds. Biomaterials, 34(17), 4266–4273.
Mackie, E. J., Ahmed, Y. A., Tatarczuch, L., Chen, K. S., & Mirams, M. (2008). Endochondral ossification: how cartilage is converted into bone in the developing skeleton. The International Journal of Biochemistry & Cell Biology, 40, 46–62.
Gadjanski, I., Spiller, K., & Vunjak-Novakovic, G. (2012). Time-dependent processes in stem cell-based tissue engineering of articular cartilage. Stem Cell Reviews, 8(3), 863–881.
Brighton C.T. (1985). Epiphyseal bone formation. In: Textbook of Small Animal Orthopaedics. Newton CD and Nunamaker DM, eds. Lippincott Williams & Wilkins, pp 50–55.
Zhu, L. L., Wu, L. Y., Yew, D. T., & Fan, M. (2005). Effects of hypoxia on the proliferation and differentiation of NSCs. Molecular Neurobiology, 31(1–3), 231–242.
Van Winkle, A. P., Gates, I. D., & Kallos, M. S. (2012). Mass transfer limitations in embryoid bodies during human embryonic stem cell differentiation. Cells, Tissues, Organs, 196(1), 34–47.
Wilson, J. L., Suri, S., Singh, A., Rivet, C. A., Lu, H., & McDevitt, T. C. (2014). Single-cell analysis of embryoid body heterogeneity using microfluidic trapping array. Biomedical Microdevices, 16(1), 79–90.
Stachelscheid, H., Wulf-Goldenberg, A., Eckert, K., Jensen, J., Edsbagge, J., Bjorquist, P., et al. (2013). Teratoma formation of human embryonic stem cells in three-dimensional perfusion culture bioreactors. Journal of Tissue Engineering and Regenerative Medicine, 7(9), 729–741.
Yamashita, A., Krawetz, R., & Rancourt, D. E. (2009). Loss of discordant cells during micro-mass differentiation of embryonic stem cells into the chondrocyte lineage. Cell Death and Differentiation, 16(2), 278–286.
Schipani, E. (2010). Posttranslational modifications of collagens as targets of hypoxia and Hif-1alpha in endochondral bone development. Annals of the New York Academy of Sciences, 1192, 317–321.
Gilkes, D. M., Bajpai, S., Chaturvedi, P., Wirtz, D., & Semenza, G. L. (2013). Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. Journal of Biological Chemistry, 288(15), 10819–10829.
Eisinger-Mathason, T. S., Zhang, M., Qiu, Q., Skuli, N., Nakazawa, M. S., Karakasheva, T., et al. (2013). Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer Discov, 3(10), 1190–1205.
Oberlender, S. A., & Tuan, R. S. (1994). Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development, 120(1), 177–187.
Ornitz, D. M., & Marie, P. J. (2002). FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes and Development, 16(12), 1446–1465.
Butler, W. T. (1989). The nature and significance of osteopontin. Connective Tissue Research, 23(2–3), 123–136.
Goldring, M. B., Tsuchimochi, K., & Ijiri, K. (2006). The control of chondrogenesis. Journal of Cellular Biochemistry, 97(1), 33–44.
Acknowledgments
We gratefully acknowledge research funding received from the NIH (grants DE016525, EB002520 and AR061988 to GVN), New York Stem Cell Foundation (NYSCF-Helmsley Investigator award to DM; grant CU09-3055 to GVN), the Ministry of Education and Science of Serbia (grants ON174028 and III41007 to IG) and Royal Thai Graduate Fellowship (to SY). We thank Dr. Jenny Yuan and Chandhanarat Chandhanayingyong for their help with animal studies.
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 604 kb)
Rights and permissions
About this article
Cite this article
Yodmuang, S., Marolt, D., Marcos-Campos, I. et al. Synergistic Effects of Hypoxia and Morphogenetic Factors on Early Chondrogenic Commitment of Human Embryonic Stem Cells in Embryoid Body Culture. Stem Cell Rev and Rep 11, 228–241 (2015). https://doi.org/10.1007/s12015-015-9584-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-015-9584-x