Abstract
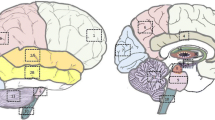
The role of selenium in human brain physiology, as well as in aging and neurodegenerative processes, remains unclear. Thus, the aim of this study was to establish the “normal” (reference) levels for selenium in the human brain, as well as anatomical regional differences and age-related changes. Using inductively coupled plasma-mass spectrometry after sample microwave-assisted acid digestion, selenium levels were measured in 14 different areas of the brain of adult individuals (n = 42; 71 ± 12, range 50–101 years old) without a known history of neurodegenerative, neurological, or psychiatric disorders. In the whole data set (n = 588; 42 individuals × 14 brain areas), selenium levels ranged from 552 to 1435 ng/g, with a mean ± SD content of 959 ± 178 ng/g (dry weight basis). Selenium distribution across the different brain areas was heterogeneous, with the highest levels in the putamen, parietal inferior lobule, and occipital cortex and the lowest expression in the medulla and cerebellum. Selenium levels were unchanged with aging. Compared with the age-matched control group, significantly increased levels of selenium were found in the globus pallidus, superior temporal gyrus, and frontal cortex of Parkinson’s disease (n = 1) and Alzheimer’s disease (n = 2) patients. This study provides new data on the anatomical regional differences in selenium levels in the human brain, which will aid future interpretation of studies examining brain tissue affected by neurodegenerative (and other) brain diseases.




Similar content being viewed by others
References
Papp LV, Lu J, Holmgren A, Khanna KK (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal 9(7):775–806
Rotruck JT, Pope AL, Ganther HE et al (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science (New York, NY) 179(4073):588–590
Chu FF, Doroshow JH, Esworthy RS (1993) Expression, characterization, and tissue distribution of a new cellular selenium-dependent glutathione peroxidase, GSHPx-GI. J Biol Chem 268(4):2571–2576
Brown KM, Arthur JR (2001) Selenium, selenoproteins and human health: a review. Public Health Nutr 4(2b):593–599
Avissar N, Ornt DB, Yagil Y et al (1994) Human kidney proximal tubules are the main source of plasma glutathione peroxidase. Am J Physiol 266(2 Pt 1):C367–375
Ursini F, Maiorino M, Gregolin C (1985) The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim Biophys Acta 839(1):62–70
Zhang S, Rocourt C, Cheng WH (2010) Selenoproteins and the aging brain. Mech Ageing Dev 131(4):253–260
Burk RF, Hill KE, Motley AK et al (2014) Selenoprotein P and apolipoprotein E receptor-2 interact at the blood-brain barrier and also within the brain to maintain an essential selenium pool that protects against neurodegeneration. FASEB J 28(8):3579–3588
Berry MJ, Larsen PR (1992) The role of selenium in thyroid hormone action. Endocr Rev 13(2):207–219
Arthur JR, McKenzie RC, Beckett GJ (2003) Selenium in the immune system. J Nutr 133(5 Suppl 1):1457S–1459S
Berr C, Arnaud J, Akbaraly TN (2012) Selenium and cognitive impairment: a brief-review based on results from the EVA study. Biofactors 38(2):139–144
Byrns CN, Pitts MW, Gilman CA, Hashimoto AC, Berry MJ (2014) Mice lacking selenoprotein P and selenocysteine lyase exhibit severe neurological dysfunction, neurodegeneration, and audiogenic seizures. J Biol Chem 289(14):9662–9674
Mehta SL, Kumari S, Mendelev N, Li PA (2012) Selenium preserves mitochondrial function, stimulates mitochondrial biogenesis, and reduces infarct volume after focal cerebral ischemia. BMC Neurosci 13:79
Steinbrenner H, Sies H (2013) Selenium homeostasis and antioxidant selenoproteins in brain: implications for disorders in the central nervous system. Arch Biochem Biophys 536(2):152–157
Caito SW, Milatovic D, Hill KE et al (2011) Progression of neurodegeneration and morphologic changes in the brains of juvenile mice with selenoprotein P deleted. Brain Res 1398:1–12
Chen J, Berry MJ (2003) Selenium and selenoproteins in the brain and brain diseases. J Neurochem 86(1):1–12
Naziroglu M (2007) New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem Res 32(11):1990–2001
Ozmen I, Naziroglu M, Alici HA et al (2007) Spinal morphine administration reduces the fatty acid contents in spinal cord and brain by increasing oxidative stress. Neurochem Res 32(1):19–25
Naziroglu M (2009) Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem Res 34(12):2181–2191
Kutluhan S, Naziroglu M, Celik O, Yilmaz M (2009) Effects of selenium and topiramate on lipid peroxidation and antioxidant vitamin levels in blood of pentylentetrazol-induced epileptic rats. Biol Trace Elem Res 129(1–3):181–189
Behl C, Moosmann B (2002) Oxidative nerve cell death in Alzheimer’s disease and stroke: antioxidants as neuroprotective compounds. Biol Chem 383(3–4):521–536
Loef M, Schrauzer GN, Walach H (2011) Selenium and Alzheimer’s disease: a systematic review. J Alzheimers Dis 26(1):81–104
Longnecker MP, Stram DO, Taylor PR et al (1996) Use of selenium concentration in whole blood, serum, toenails, or urine as a surrogate measure of selenium intake. Epidemiol (Cambridge, Mass) 7(4):384–390
Ashton K, Hooper L, Harvey LJ et al (2009) Methods of assessment of selenium status in humans: a systematic review. Am J Clin Nutr 89(6):2025s–2039s
Segovia G, Porras A, Del Arco A, Mora F (2001) Glutamatergic neurotransmission in aging: a critical perspective. Mech Ageing Dev 122(1):1–29
Paine SML, Lowe JS (2011) Approach to the post-mortem investigation of neurodegenerative diseases: from diagnosis to research. Diagn Histopathol 17(5):211–216
Duflou H, Maenhaut W, De Reuck J (1989) Regional distribution of potassium, calcium, and six trace elements in normal human brain. Neurochem Res 14(11):1099–1112
Lanciego JL, Luquin N, Obeso JA (2012) Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med 2(12):a009621
Ramos P, Santos A, Pinto NR et al (2014) Iron levels in the human brain: a post-mortem study of anatomical region differences and age-related changes. J Trace Elem Med Biol 28(1):13–17
Larsen NA, Pakkenberg H, Damsgaard E, Heydorn K (1979) Topographical distribution of arsenic, manganese, and selenium in the normal human brain. J Neurol Sci 42(3):407–416
Hock A, Demmel U, Schicha H, Kasperek K, Feinendegen LE (1975) Trace element concentration in human brain. Activation analysis of cobalt, iron, rubidium, selenium, zinc, chromium, silver, cesium, antimony and scandium. Brain 98(1):49–64
Ejima A, Watanabe C, Koyama H, Matsuno K, Satoh H (1996) Determination of selenium in the human brain by graphite furnace atomic absorption spectrometry. Biol Trace Elem Res 54(1):9–21
Behne D, Wolters W (1983) Distribution of selenium and glutathione peroxidase in the rat. J Nutr 113(2):456–461
Kühbacher M, Bartel Jr, Alber D et al (2014) Neurochemical imaging of selenium homeostasis and the selenoproteome in the brain under nutritional deficiency. http://www.laborjournal.de/editorials/ed424/Ms1_v5_web.pdf. Accessed 21st August 2014
Markesbery WR, Ehmann WD, Alauddin M, Hossain TI (1984) Brain trace element concentrations in aging. Neurobiol Aging 5(1):19–28
Nakayama A, Hill KE, Austin LM, Motley AK, Burk RF (2007) All regions of mouse brain are dependent on selenoprotein P for maintenance of selenium. J Nutr 137(3):690–693
Brannan TS, Maker HS, Raes I, Weiss C (1980) Regional distribution of glutathione reductase in the adult rat brain. Brain Res 200(2):474–477
Zhang Y, Zhou Y, Schweizer U et al (2008) Comparative analysis of selenocysteine machinery and selenoproteome gene expression in mouse brain identifies neurons as key functional sites of selenium in mammals. J Biol Chem 283(4):2427–2438
Hebbrecht G, Maenhaut W, Reuck JD (1999) Brain trace elements and aging. Nucl Instrum Meth B 150(1–4):208–213
Bartzokis G, Tishler TA, Lu PH et al (2007) Brain ferritin iron may influence age- and gender-related risks of neurodegeneration. Neurobiol Aging 28(3):414–423
Xu X, Wang Q, Zhang M (2008) Age, gender, and hemispheric differences in iron deposition in the human brain: an in vivo MRI study. NeuroImage 40(1):35–42
Correia H, Ramos P, Santos A et al (2014) A post-mortem study of the anatomical region differences and age-related changes on Ca and Mg levels in the human brain. Microchem J 113:69–76
Acknowledgments
This work received financial support from the Universidade do Porto and Santander Totta through the project “TRAIN: Trace elements in human brain: age-related changes and anatomic region specific differences” (PP_IJUP 2011 342). This work also received financial support from the European Union (FEDER funds through COMPETE) and national funds (FCT, Fundação para a Ciência e Tecnologia) through project Pest-C/EQB/LA0006/2013. The authors thank all financing sources.
Conflict of Interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramos, P., Santos, A., Pinto, N.R. et al. Anatomical Regional Differences in Selenium Levels in the Human Brain. Biol Trace Elem Res 163, 89–96 (2015). https://doi.org/10.1007/s12011-014-0160-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0160-z