Abstract
Background
Dexamethasone is a potent analgesic and antiemetic. However, the benefit of dexamethasone after TKA is unclear, as is the efficacy in a current multimodal regime.
Questions/purposes
We determined (1) whether the addition of dexamethasone to a protocol including ramosetron further reduces postoperative emesis compared with ramosetron alone; (2) whether it reduces postoperative pain; and (3) whether it increases the risk for wound complications in a current multimodal regime after TKA.
Methods
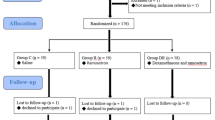
We randomized 269 patients undergoing TKAs to receive dexamethasone (10 mg) 1 hour before surgery and ramosetron immediately after surgery (Dexa-Ra group, n = 135), or ramosetron alone (Ra group, n = 134). We recorded the incidence of postoperative nausea and vomiting (PONV), severity of nausea, incidence of antiemetic requirement, complete response, pain level, and opioid consumption. Patients were assessed 0 to 6, 6 to 24, 24 to 48, and 48 to 72 hours postoperatively. In addition, patients were evaluated for wound complications and periprosthetic joint infections at a minimum of 1 year after surgery.
Results
The Dexa-Ra group had a lower incidence of PONV during the entire 72-hour evaluation period and experienced less severe nausea for the first 6 hours after TKA, although not between 6 to 72 hours. Overall use of a rescue antiemetic was less frequent, and complete response was more frequent in the Dexa-Ra group. Patients in the Dexa-Ra group experienced lower pain and consumed less opioids during the 6- to 24-hour period and during the overall study period. No differences were found in wound complications between the groups, and each group had one case of periprosthetic joint infection.
Conclusions
Patients who received prophylactic dexamethasone in addition to ramosetron had reduced postoperative emesis and pain without increased risks for wound complications, compared with patients who received ramosetron alone in patients managed using a multimodal regimen after TKA.
Level of Evidence
Level I, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.




Similar content being viewed by others
References
Aminmansour B, Khalili HA, Ahmadi J, Nourian M. Effect of high-dose intravenous dexamethasone on postlumbar discectomy pain. Spine (Phila Pa 1976). 2006;31:2415–2417.
Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, Zernak C, Danner K, Jokela R, Pocock SJ, Trenkler S, Kredel M, Biedler A, Sessler DI, Roewer N. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004;350:2441–2451.
Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700.
Apfel CC, Roewer N, Korttila K. How to study postoperative nausea and vomiting. Acta Anaesthesiol Scand. 2002;46:921–928.
Bergeron SG, Kardash KJ, Huk OL, Zukor DJ, Antoniou J. Perioperative dexamethasone does not affect functional outcome in total hip arthroplasty. Clin Orthop Relat Res. 2009;467:1463–1467.
Callahan CM, Drake BG, Heck DA, Dittus RS. Patient outcomes following tricompartmental total knee replacement: a meta-analysis. JAMA. 1994;271:1349–1357.
Chang RW, Pellisier JM, Hazen GB. A cost-effectiveness analysis of total hip arthroplasty for osteoarthritis of the hip. JAMA. 1996;275:858–865.
Chen JJ, Frame DG, White TJ. Efficacy of ondansetron and prochlorperazine for the prevention of postoperative nausea and vomiting after total hip replacement or total knee replacement procedures: a randomized, double-blind, comparative trial. Arch Intern Med. 1998;158:2124–2128.
De Oliveira GS Jr, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115:575–588.
DiIorio TM, Sharkey PF, Hewitt AM, Parvizi J. Antiemesis after total joint arthroplasty: does a single preoperative dose of aprepitant reduce nausea and vomiting? Clin Orthop Relat Res. 2010;468:2405–2409.
Dorr LD, Chao L. The emotional state of the patient after total hip and knee arthroplasty. Clin Orthop Relat Res. 2007;463:7–12.
Edwards CL, Fillingim RB, Keefe F. Race, ethnicity and pain. Pain. 2001;94:133–137.
Filos KS, Lehmann KA. Current concepts and practice in postoperative pain management: need for a change? Eur Surg Res. 1999;31:97–107.
Fortin PR, Clarke AE, Joseph L, Liang MH, Tanzer M, Ferland D, Phillips C, Partridge AJ, Belisle P, Fossel AH, Mahomed N, Sledge CB, Katz JN. Outcomes of total hip and knee replacement: preoperative functional status predicts outcomes at six months after surgery. Arthritis Rheum. 1999;42:1722–1728.
Fujii Y, Nakayama M. Effects of dexamethasone in preventing postoperative emetic symptoms after total knee replacement surgery: a prospective, randomized, double-blind, vehicle-controlled trial in adult Japanese patients. Clin Ther. 2005;27:740–745.
Gan TJ. Postoperative nausea and vomiting: can it be eliminated? JAMA. 2002;287:1233–1236.
Gan TJ. Risk factors for postoperative nausea and vomiting. Anesth Analg. 2006;102:1884–1898.
Gan TJ, Meyer T, Apfel CC, Chung F, Davis PJ, Eubanks S, Kovac A, Philip BK, Sessler DI, Temo J, Tramer MR, Watcha M. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003;97:62–71.
Gilron I. Corticosteroids in postoperative pain management: future research directions for a multifaceted therapy. Acta Anaesthesiol Scand. 2004;48:1221–1222.
Hahm TS, Ko JS, Choi SJ, Gwak MS. Comparison of the prophylactic anti-emetic efficacy of ramosetron and ondansetron in patients at high-risk for postoperative nausea and vomiting after total knee replacement. Anaesthesia. 2010;65:500–504.
Henzi I, Walder B, Tramer MR. Dexamethasone for the prevention of postoperative nausea and vomiting: a quantitative systematic review. Anesth Analg. 2000;90:186–194.
Holte K, Kehlet H. Perioperative single-dose glucocorticoid administration: pathophysiologic effects and clinical implications. J Am Coll Surg. 2002;195:694–712.
Jules-Elysee KM, Lipnitsky JY, Patel N, Anastasian G, Wilfred SE, Urban MK, Sculco TP. Use of low-dose steroids in decreasing cytokine release during bilateral total knee replacement. Reg Anesth Pain Med. 2011;36:36–40.
Jules-Elysee KM, Wilfred SE, Memtsoudis SG, Kim DH, Yadeau JT, Urban MK, Lichardi ML, McLawhorn AS, Sculco TP. Steroid modulation of cytokine release and desmosine levels in bilateral total knee replacement: aprospective, double-blind, randomized controlled trial. J Bone Joint Surg Am. 2012;94:2120–2127.
Kardash KJ, Sarrazin F, Tessler MJ, Velly AM. Single-dose dexamethasone reduces dynamic pain after total hip arthroplasty. Anesth Analg. 2008;106:1253–1257.
Kim S. Changes in surgical loads and economic burden of hip and knee replacements in the US: 1997–2004. Arthritis Rheum. 2008;59:481–488.
Koh IJ, Chang CB, Jeon YT, Ryu JH, Kim TK. Does ramosetron reduce postoperative emesis and pain after TKA? Clin Orthop Relat Res. 2012;470:1718–1727.
Koh IJ, Chang CB, Seo ES, Kim SJ, Seong SC, Kim TK. Pain management by periarticular multimodal drug injection after anterior cruciate ligament reconstruction: a randomized, controlled study. Arthroscopy. 2012;28:649–657.
Koh IJ, Kang YG, Chang CB, Do SH, Seong SC, Kim TK. Does periarticular injection have additional pain relieving effects during contemporary multimodal pain control protocols for TKA?: a randomised, controlled study. Knee. 2012;19:253–259.
Koh IJ, Kang YG, Chang CB, Kwon SK, Seo ES, Seong SC, Kim TK. Additional pain relieving effect of intraoperative periarticular injections after simultaneous bilateral TKA: a randomized, controlled study. Knee Surg Sports Traumatol Arthrosc. 2010;18:916–922.
Koh IJ, Kim TK, Chang CB, Cho HJ, In Y. Trends in use of total knee arthroplasty in Korea from 2001 to 2010. Clin Orthop Relat Res. 2013;471:1441–1450.
Koivuranta M, Laara E, Snare L, Alahuhta S. A survey of postoperative nausea and vomiting. Anaesthesia. 1997;52:443–449.
Korttila K. The study of postoperative nausea and vomiting. Br J Anaesth. 1992;69(7 suppl 1):20S–23S.
Kovac AL. Prevention and treatment of postoperative nausea and vomiting. Drugs. 2000;59:213–243.
Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785.
Lee Y, Lin YS, Chen YH. The effect of dexamethasone upon patient-controlled analgesia-related nausea and vomiting. Anaesthesia. 2002;57:705–709.
Lombardi AV Jr, Berend KR, Mallory TH, Dodds KL, Adams JB. Soft tissue and intra-articular injection of bupivacaine, epinephrine, and morphine has a beneficial effect after total knee arthroplasty. Clin Orthop Relat Res. 2004;428:125–130.
Lunn TH, Kristensen BB, Andersen LO, Husted H, Otte KS, Gaarn-Larsen L, Kehlet H. Effect of high-dose preoperative methylprednisolone on pain and recovery after total knee arthroplasty: a randomized, placebo-controlled trial. Br J Anaesth. 2011;106:230–238.
Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg. 1999;89:652–658.
Miyagawa Y, Ejiri M, Kuzuya T, Osada T, Ishiguro N, Yamada K. Methylprednisolone reduces postoperative nausea in total knee and hip arthroplasty. J Clin Pharm Ther. 2010;35:679–684.
Myles PS, Williams DL, Hendrata M, Anderson H, Weeks AM. Patient satisfaction after anaesthesia and surgery: results of a prospective survey of 10,811 patients. Br J Anaesth. 2000;84:6–10.
Park KK, Kim TK, Chang CB, Yoon SW, Park KU. Normative temporal values of CRP and ESR in unilateral and staged bilateral TKA. Clin Orthop Relat Res. 2008;466:179–188.
Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93:1075–1084.
Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–2994.
Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids: new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723.
Salerno A, Hermann R. Efficacy and safety of steroid use for postoperative pain relief. update and review of the medical literature. J Bone Joint Surg Am. 2006;88:1361–1372.
Shen H, Zhang N, Zhang X, Ji W. C-reactive protein levels after 4 types of arthroplasty. Acta Orthop. 2009;80:330–333.
Sinatra RS, Torres J, Bustos AM. Pain management after major orthopaedic surgery: current strategies and new concepts. J Am Acad Orthop Surg. 2002;10:117–129.
Smith C, Erasmus PJ, Myburgh KH. Endocrine and immune effects of dexamethasone in unilateral total knee replacement. J Int Med Res. 2006;34:603–611.
Song KH, Kang YM, Sin HY, Yoon SW, Seo HK, Kwon S, Shin MJ, Chang CB, Kim TK, Kim HB. Outcome of cefazolin prophylaxis for total knee arthroplasty at an institution with high prevalence of methicillin-resistant Staphylococcus aureus infection. Int J Infect Dis. 2011;15:e867–870.
Watcha MF, White PF. Postoperative nausea and vomiting: its etiology, treatment, and prevention. Anesthesiology. 1992;77:162–184.
Acknowledgments
We thank Jung-Hee Ryu MD of the Department of Anesthesiology and Pain Medicine for anesthesia and Yeon Gwi Kang MS of Seoul National University Bundang Hospital for data collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution has approved the human protocol for this
investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained.
This work was performed at the Joint Reconstruction Center, Seoul National University Bundang Hospital, Seongnam-si, Korea.
About this article
Cite this article
Koh, I.J., Chang, C.B., Lee, J.H. et al. Preemptive Low-dose Dexamethasone Reduces Postoperative Emesis and Pain After TKA: A Randomized Controlled Study. Clin Orthop Relat Res 471, 3010–3020 (2013). https://doi.org/10.1007/s11999-013-3032-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-013-3032-5