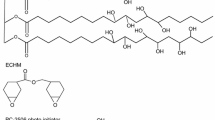
Abstract
Di-hydroxylated soybean oil (DSO), a biobased polyol synthesized from epoxidized soybean oil (ESO) could be used to formulate resins for adhesives; however, current DSO synthesis requires harsh reaction conditions that significantly increase both cost and waste generation. In this paper, we investigate the kinetics of oxirane cleavage in ESO to DSO by water and elucidate the role of different process parameters in the reaction rate and optimization of reaction conditions. Our kinetic study showed that ESO oxirane cleavage was a first-order reaction and that the ESO oxirane cleavage rate was greatly influenced by tetrahydrofuran (THF)/ESO ratio, H2O/ESO ratio, catalyst content, and temperature. Optimized reaction parameters were THF/ESO of 0.5, H2O/ESO of 0.25, catalyst content of 1.5 %, and reaction time of 3 h at 25 °C. DSO with hydroxyl value of 242 mg KOH/g was obtained under these conditions. We also characterized the structure, thermal properties, adhesion performance, and viscoelasticity of UV-polymerized resins based on this DSO. The resin tape exhibited peel adhesion strength of 3.6 N/in., which is comparable to some commercial tapes measured under similar conditions.










Similar content being viewed by others
References
Desroches M, Escouvois M, Auvergne R, Caillol S, Boutevin B (2012) From vegetable oils to polyurethanes: synthetic routes to polyols and main industrial products. Polym Rev 52:38–79
Petrovic ZS (2008) Polyurethanes from vegetable oils. Polym Rev 48:109–155
Lligadas G, Ronda JC, Galia M, Cadiz V (2010) Plant oils as platform chemicals for polyurethane synthesis: current state-of-the-art. Biomacromolecules 11:2825–2835
Zaher F, Elmallah M, Elhefnawy M (1989) Kinetics of oxirane cleavage in epoxidized soybean oil. J Am Oil Chem Soc 66:698–700
Zaher F, Elshami S (1990) Oxirane ring-opening by formic acid. Grasas Aceites 41:361–365
Lin B, Yang L, Dai H, Yi A (2008) Kinetic studies on oxirane cleavage of epoxidized soybean oil by methanol and characterization of polyols. J Am Oil Chem Soc 85:113–117
Guo R, Ma C, Sun S, Ma Y (2011) Kinetic study on oxirane cleavage of epoxidized palm oil. J Am Oil Chem Soc 88:517–521
Ahn BK, Kraft S, Wang D, Sun XS (2011) Thermally transparent, pressure-sensitive adhesives from epoxidized and dihydroxyl soybean oil, stable. Biomacromolecules 12:1839–1843
Ahn B, Sung J, Kim N, Kraft S, Sun X (2013) UV-curable pressure-sensitive adhesives derived from functionalized soybean oils and rosin ester. Polym Int 62:1293–1301
Adhvaryu A, Liu Z, Erhan S (2005) Synthesis of novel alkoxylated triacylglycerols and their lubricant base oil properties. Ind Crop Prod 21:113–119
Zhao H, Zhang J, Sun X, Hua D (2008) Syntheses and properties of cross-linked polymers from functionalized triglycerides. J Appl Polym Sci 110:647–656
Guo Y, Hardesty J, Mannari V, Massingill J Jr (2007) Hydrolysis of epoxidized soybean oil in the presence of phosphoric acid. J Am Oil Chem Soc 84:929–935
Kong X, Liu G, Qi H, Curtis JM (2013) Preparation and characterization of high-solid polyurethane coating systems based on vegetable oil derived polyols. Prog Org Coat 76:1151–1160
Caillol S, Desroches M, Boutevin G, Loubat C, Auvergne R, Boutevin B (2012) Synthesis of new polyester polyols from epoxidized vegetable oils and biobased acids. Eur J Lipid Sci Technol 114:1447–1459
Park S, Jin F, Lee J (2004) Synthesis and thermal properties of epoxidized vegetable oil. Macromol Rapid Commun 25:724–727
Chakrapani S, Crivello J (1998) Synthesis and photoinitiated cationic polymerization of epoxidized castor oil and its derivatives. J Macromol Sci: Pure Appl Chem A35:1–20
Li Y, Sun X (2014) Di-hydroxylated soybean oil polyols with varied hydroxyl values and their influence on UV-curable pressure-sensitive adhesives. J Am Oil Chem Soc 91:1425–1432
Chang EP (1997) Viscoelastic properties of pressure-sensitive adhesives. J Adhes 60:233–248
Yang H, Chang E (1997) The role of viscoelastic properties in the design of pressure-sensitive adhesives. Trends Polymer Sci 5:380–384
Dahlquist CA (1966) Tack. In: Eley DD (ed) Adhesion fundamentals and practice. McLaren, London, pp. 143–151
Acknowledgments
Contribution No. 14-103-J from the Kansas Agricultural Experimental Station. Financial support was provided by Kansas Soybean Commission, United Soybean Board, and Henkel.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Li, Y., Wang, D. & Sun, X.S. Oxirane Cleavage Kinetics of Epoxidized Soybean Oil by Water and UV-Polymerized Resin Adhesion Properties. J Am Oil Chem Soc 92, 121–131 (2015). https://doi.org/10.1007/s11746-014-2564-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-014-2564-5