Abstract
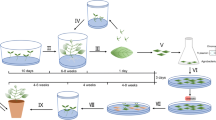
In vitro regeneration of Parkia timoriana (DC.) Merr. has been achieved using cotyledonary node explants. The ability to produce multiple shoots has been evaluated using semi-solid Murashige and Skoog (MS) basal medium and Gamborg’s B-5 basal medium supplemented with various concentrations of α-naphthalene acetic acid (NAA) and 6-benzylaminopurine (BA) either in single or in combinations. The explants cultured in MS medium supplemented with combinations of 2.7 μM NAA and 11 μM BA showed the maximum frequency of multiple shoots (96.66%) formation and number of shoots per explants (6.60), respectively. For rooting, full and half strength MS medium supplemented with various concentrations of indole-3-butyric acid (IBA) and NAA were studied and the highest number of root formation was observed in full-strength MS supplemented with 9.8 μM IBA. Using Agrobacterium tumefaciens strain EHA105 pCAMBIA2301 various optimum conditions for efficient transformation were determined by recording the percentage of GUS+ explants. Following the optimized conditions, the co-cultured explants were cultured on semi-solid shoot regeneration medium containing MS medium + 2.7 μM NAA + 11 μM BA + 100 mg/l kanamycin + 500 mg/l cefotaxime. After 8 weeks of culture, the regenerated shoots were rooted in rooting medium (RM) containing MS medium + 9.8 μM indole-3-butyric acid (IBA), 3% sucrose, 7.5 mg/l kanamycin and 500 mg/l cefotaxime. Successful transformation was confirmed by histochemical GUS activity of the regenerated shoots, nptII gene PCR analyses of the regenerated kanamycin resistant plantlets and Southern analysis of putative transgenic PCR+ plants.

Similar content being viewed by others
Abbreviations
- MS:
-
Murashige and Skoog (1962)
- B-5:
-
Gamborg et al. (1968)
- NAA:
-
α-Naphthalene acetic acid
- BA:
-
6-Benzylaminopurine
- IBA:
-
Indole-3-butyric acid
- GUS:
-
β-Glucuronidase
- CaMV:
-
35S 35S promoter of the cauliflower mosaic virus
- nptII:
-
Neomycin phosphotransferase
- PCR:
-
Polymerase chain reaction
- OD600 :
-
Optical density at 600 nm
References
Ajithkumar D, Seeni S (1998) Rapid cloncal multiplication through in vitro axillary shoot proliferation of Aegle marmelos (L.) Corr. a medicinal tree. Plant Cell Rep 17:422–426. doi:10.1007/s002990050418
Al-Wasel AS (2000) Micropropagation of Acacia seyal Del. in vitro. J Arid Environ 46:425–431. doi:10.1006/jare.2000.0687
Amoo SO, Ayisire BE (2005) Induction of callus and somatic embryogenesis from cotyledon explants of Parkia biglobosa (Jacq.) Benth. Afr J Biotechnol 4:68–71
Arumugam S, Rao MV (1996) In vitro production of plantlets from cotyledonary node cultures of Aegle marmelos (L.) Corr. Adv Plant Sci 9:181–186
Baulcombe D (2004) RNA silencing in plants. Nature 431:356–363
Cervera M, Ortega C, Navarro A, Navarro L, Pena L (2000) Generation of transgenic citrus plants with tolerance-to-salinity gene HAL2 from yeast. J Hortic Sci Biotechnol 75:26–30
Chilton MD, Currier TC, Farrand SK, Bendich AJ, Gordon MP, Nestor EW (1974) Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc Natl Acad Sci USA 71:3672–3676
Datta SK, Datta K (1983) Auxin induced regeneration of forest tree—Dalbergia sissoo Roxb. through tissue culture. Curr Sci 52:434–436
Dewan A, Nanda K, Gupta SC (1992) In vitro micropropagation of Acacia nilotica sub sp indica Brenan via cotyledonary nodes. Plant Cell Rep 12:18–21. doi:10.1007/BF00232415
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements for superior cultures of soybean root cells. Exp Cell Res 50:151–158. doi:10.1016/0014-4827(68)90403-5
Hopkins HCF (1994) The Indo-Pacific species of Parkia (Leguminosae: Mimosoideae). Kew Bull 49:181–234
Hossain M, Islam R, Karim MR, Joarder OI, Biswas BK (1994) Regeneration of plantlets from in vitro cultured cotyledons of Aegle marmelos Corr (Rutaceae). Sci Hort 57:315–321. doi:10.1016/0304-4238(94)90114-7
Jefferson RA (1987) Assaying chimeric in plants: the GUS gene fusion system. Plant Mol Bio Rep 5:387–405. doi:10.1007/BF02667740
Jaiwal PK, Kumari R, Ignacimuthu S, Potrykus I, Sautter C (2001) Agrobacterium tumfefaciens-mediated genetic transformation of mungbean (Vigna radiata L. Wilczek)—a recalcitrant grain legume. Plant Sci 161:239–247. doi:10.1016/S0168-9452(01)00352-1
Joshi I, Bisht P, Sharma VK, Uniyal DP (2003) Studies on effect of nutrient media for clonal propagation of superior phenotypes of Dalbergia sissoo Roxb. through tissue culture. Silvae Genetica 52:3–4
Kanjilal UN, Kanjilal PC, Das A (1982) Flora of Assam, vol 2. Avon, Delhi, p 151
Karthikeyan AS, Sarma KS, Veluthambi K (1996) Agrobacterium tumefaciens-mediated transformation of Vigna mungo L. Hepper. Plant Cell Rep 15:328–331. doi:10.1007/BF00232365
Longvah T, Deosthale YG (1998) Nutrient composition and food potential of Parkia roxburghii, a less known tree legume from northeast India. Food Chem 62:477–481. doi:10.1016/S0308-8146(97)00179-9
Mittal A, Agarwal R, Gupta SC (1989) In vitro developments of plantlets from axillary buds of Acacia auriculiformis—a leguminous tree. Plant Cell Tissue Org Cult 19:55–60. doi:10.1007/BF00037777
Moore GA, Jacomo CC, Neidigh JL, Lawrence SD, Cline K (1992) Agrobacterium-mediated transformation of Citrus stem segments and regeneration of transgenic plants. Plant Cell Rep 11:238–242. doi:10.1007/BF00235073
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–491. doi:10.1111/j.1399-3054.1962.tb08052.x
Pradhan C, Kar S, Pattnaik S, Chand PK (1998) Propagation of Dalbergia sissoo Roxb through in vitro shoot proliferation from cotyledonary nodes. Plant Cell Rep 18:122–126. doi:10.1007/s002990050543
Perez-Molphe-Balch E, Ochoa-Alejo N (1998) Regeneration of transgenic plants of Mexican lime from Agrobacterium rhizogenes-transformed tissues. Plant Cell Rep 17:591–596. doi:10.1007/s002990050448
Purohit SD, Dave A (1996) Micropropagation of Sterculia urens Roxb an endangered tree species. Plant Cell Rep 15:704–706. doi:10.1007/BF00231929
Saklani A, Rao RR (2002) In: Rao (ed.) Advances in legume research in India. Bishen Singh Mahendra Pal Singh, Dehra Dun, pp 239–250
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sharma A, Haridasan K, Borthakur K (2002) In: Rao (ed.) Advances in legume research in India. Bishen Singh Mahendra Pal Singh, Dehra Dun, pp 171–179
Suvachittanont W, Kurashima Y, Esumi H, Tsuda M (1996) Formation of thiazolidine-4-carboxylic acid (thioproline), an effective nitrite-trapping agent in human body, in Parkia speciosa seeds and other edible leguminous seeds in Thailand. Food Chem 55:359–363. doi:10.1016/0308-8146(95)00132-8
Tahira T, Tsuda M, Wakabayashi K, Nagao M, Sugumura T (1984) Kinetics of nitrosation of a major nitroso compound in human urine and its role as nitrite scavenger. Gann 75:889–894
Tahira T, Ohgaki H, Wakabayashi K, Nagao M, Sugumura T (1988) Inhibitory effect of thioproline on carcinogenesis induced by N-benzylmethylamine and nitrite. Food Chem Toxicol 26:511–518. doi:10.1016/0278-6915(88)90003-8
Thangjam R, Damayanti M, Jitendra GS (2003a) Cadra cautella Walker (Lepidoptera: Crambidae: Phycitinae)—a pest on Parkia timoriana (DC.) Merr. in Manipur. Curr Sci 85:725–726
Thangjam R, Damayanti M, Jitendra GS (2003b) A simple and rapid method for isolation of DNA from imbibed embryos of Parkia timoriana (DC.) Merr. for PCR analysis. J Food Agric Environ 1:36–38
Thangjam R, Maibam RS (2006) Induction of callus and somatic embryogenesis of cotyledonary explants of Parkia timoriana (DC.) Merr., a multipurpose tree legume. J Food Agric Environ 4:335–339
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Moriguchi.
Rights and permissions
About this article
Cite this article
Thangjam, R., Sahoo, L. In vitro regeneration and Agrobacterium tumefaciens-mediated genetic transformation of Parkia timoriana (DC.) Merr.: a multipurpose tree legume. Acta Physiol Plant 34, 1207–1215 (2012). https://doi.org/10.1007/s11738-011-0917-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-011-0917-3