Abstract
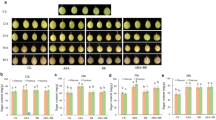
The aim of this experiment was to study the effect of 24-epibrassinolide (BR27) on fatty acids composition and sugar content in winter oilseed rape callus cultured at 20 and 5°C. Studies have showed that BR27 action is highly temperature-dependent. The increase in sugar content (sucrose, glucose and fructose) by BR27 in concentration 100 nM was observed only in calli cultured at 20°C. At 5°C, quite the opposite effect of BR27 action was observed; where cold increased the sugar content, BR27 decreased it. BR27 at 20°C had a similar effect on the fatty acid composition of phospholipids (PL) as the cold in the process of frost hardening of oilseed rape calli. BR27 decreased the 16:0, 18:1 and 18:2 and increased the 18:3 fatty acid content. At 5°C, BR27 (100 nM) generally did not influence the fatty acid composition of PL. In case of digalactosyl diacylglycerols and monogalactosyl diacylglycerols, the influence of BR27 on the fatty acid composition is ambiguous but still depends on temperature.




Similar content being viewed by others
References
Bakht J, Bano A, Dominy P (2006) The role of abscisic acid and low temperature in chickpea (Cicer arietinum) cold tolerance: II. Effects on plasma membrane structure and function. J Exp Bot 57:3707–3715. doi:10.1093/jxb/erl120
Bishop GJ, Yokota T (2001) Plants steroid hormones, brassinosteroids: current highlights of molecular aspects on their synthesis/metabolism, transport, perception and response. Plant Cell Physiol 42:114–120. doi:10.1093/pcp/pce018
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Braun P, Wild A (1984) The influence of brassinosteroid on growth and parameters of photosynthesis of wheat and mustard plants. J Plant Physiol 116:189–196
Bredemeijer GMM, Esselink G (1995) Sugar metabolism in cold-hardened Lolium perenne varieties. Plant Var Seeds 8:187–195
Dahse I, Sack H, Bernstein M, Petzold U, Müller E, Vorbrodt HM et al (1990) Effects of (22S, 23S)-homobrassinolide and related compounds on membrane potential and transport of egeria leaf cells. Plant Physiol 93:1268–1271
Dallaire S, Houde M, Gagné Y, Saini HS, Boileau S, Chevrier N et al (1994) ABA and low temperature induce freezing tolerance via distinct regulatory pathways in wheat. Plant Cell Physiol 35:1–9
Ehsanpour AA, Amini F (2003) Effect of salt and drought stress on acid phosphatase activities in alfalfa (Medicago sativa L.) explants under in vitro culture. Afr J Biotechnol 2:133–135
Eun JS, Kuraishi S, Sakurai N (1989) Changes in levels of auxin and abscisic acid and the evolution of ethylene in squash hypocotyls after treatment with brassinolide. Plant Cell Physiol 30(6):807–810
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissue. J Biol Chem 225:497–509
Gaudinová A, Sűssenbeková H, Vojtěchová M, Kaminek M, Eder J, Kohout L (1995) Different effects of two brassinosteroids on growth, auxin and cytokinin content in tobacco callus tissue. Plant Growth Regul 17:121–126. doi:10.1007/BF00024171
Grindstaff KK, Fielding LA, Brodl MR (1996) Effect of gibberellin and heat shock on the lipid composition of endoplasmic reticulum in barley aleurone layers. Plant Physiol 110:571–581
Grove MD, Spencer GF, Rohwedder WK, Mandava N, Worley JF, Warthen JD et al (1979) Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 281:216–217. doi:10.1038/281216a0
Haubrick LL, Assmann SM (2006) Brassinosteroids and plant function: some clues, more puzzles. Plant Cell Environ 29:446–457. doi:10.1111/j.1365-3040.2005.01481.x
Hurry VM, Strand Å, Tobiaeson M, Gardeström P, Öquist G (1995) Cold hardening of spring and winter wheat and rape results in differential effects on growth, carbon metabolism, and carbohydrate content. Plant Physiol 109:697–706
Janeczko A (2000) The influence of selected steroids on plant physiological processes—especially on flowering induction. Doctor Theses, Agricultural University, Krakow, Poland
Janeczko A, Kościelniak J, Pilipowicz M, Szarek-Łukaszewska G, Skoczowski A (2005) Protection of winter rape photosystem 2 by 24-epibrassinolide under cadmium stress. Photosynthetica 43:293–298. doi:10.1007/s11099-005-0048-4
Janeczko A, Gullner G, Skoczowski A, Dubert F, Barna B (2007) Effects of brassinosteroid infiltration prior to cold treatment on ion leakage and pigment contents in rape leaves. Biol Plant 51:355–358. doi:10.1007/s10535-007-0072-2
Johnson G, Williams JP (1989) Effect of growth temperature on the biosynthesis of chloroplastic galactosyldiacylglycerol molecular species in Brassica napus leaves. Plant Physiol 91:924–929
Johnson-Flanagan AM, Huiwen Z, Thiagarajah MR, Saini HS (1991) Role of abscisic acid in the induction of freezing tolerance in Brassica napus suspension-cultured cells. Plant Physiol 95:1044–1048
Krishna P (2003) Brassinosteroid-mediated stress responses. J Plant Growth Regul 22:289–297. doi:10.1007/s00344-003-0058-z
Kubacka-Zębalska M, Kacperska A (1999) Low temperature-induced modifications of cell wall content and polysaccharide composition in leaves of winter oilseed rape (Brassica napus var. oleifera L.). Plant Sci 148:59–67. doi:10.1016/S0168-9452(99)00122-3
Kuiper PJC (1985) Environmental changes and lipid metabolism of higher plants. Physiol Plant 64:118–122. doi:10.1111/j.1399-3054.1985.tb01221.x
Kull U, Kühn B, Schweizer J, Weiser H (1978) Short-term effects of cytokinins on the lipid fatty acids of green leaves. Plant Cell Physiol 19:801–810
Lee SP, Chen THH (1993) Molecular cloning of abscisic acid-responsive messenger RNAs expressed during the induction of freezing tolerance in Bromegrass (Bromus inermis Leyss) suspension culture. Plant Physiol 101:1089–1096. doi:10.1104/pp.101.3.1089
Liu X, Huang B (2002) Cytokinin effects on creeping bentgrass response to heat stress. II. Leaf senescence and antioxidant metabolism. Crop Sci 42:466–472
Liu W, Hildebrand DF, Collins GB (1995) Auxin-regulated changes of fatty acid content and composition in soybean zygotic embryo cotyledons. Plant Sci 106:31–42. doi:10.1016/0168-9452(95)04067-5
Lu Z, Huang M, Ge DP, Yang YH, Cai XN, Qin P et al (2003) Effect of brassinolide on callus growth and regeneration in Spartina patens (Poaceae). Plant Cell Tissue Organ Cult 73:87–89. doi:10.1023/A:1022665210113
Mandava NB (1988) Plant growth-promoting brassinosteroids. Annu Rev Plant Physiol Plant Mol Biol 39:23–52. doi:10.1146/annurev.pp.39.060188.000323
Mazliak P (1977) Glyco- and phospholipids of biomembranes in higher plants. In: Tevini M, Lichtenthaler HK (eds) Lipids and lipid polymers in higher plants, vol 3. Springer, Berlin, pp 48–72
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with plant tissue culture. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Núñez M, Siqueira WJ, Hernández M, Zullo MAT, Robaina C, Coll F (2004) Effect of spirostane analogues of brassinosteroids on callus formation and plant regeneration in lettuce (Lactuca sativa). Plant Cell Tissue Organ Cult 78:97–99. doi:10.1023/B:TICU.0000020400.79230.c7
Ono EO, Nakamura T, Machado SR, Rodrigues JD (2000) Application of brassinosteroid to Tabebuia alba (Bignoniaceae) plants. Rev Bras Fisiol Vegetal 12:187–194. doi:10.1590/S0103-31312000000300002
Pontis HG (1989) Fructans and cold stress. J Plant Physiol 134:148–150
Rapacz M, Dawid K (1999) An opportunity for fast and reliable evaluation of winter oilseed rape frost resistance using in vitro cultures. J Agron Crop Sci 182:193–198. doi:10.1046/j.1439-037x.1999.00282.x
Rivera CM, Penner D (1978) Rapid changes in soybean root membrane lipids with altered temperature. Phytochemistry 17:1269–1272. doi:10.1016/S0031-9422(00)94570-9
Robertson AJ, Ishikawa M, Gusta LV (1995) The effect of prolonged abscisic acid treatment on the growth, freezing tolerance and protein patterns of Bromus inermis (Leyss) cell suspensions cultured at either 3 degrees or 25 degrees C. J Plant Physiol 145:137–142
Ryyppö A, Vapaavuori EM, Rikala R, Sutinen ML (1994) Fatty acid composition of microsomal phospholipids and H+-ATP-ase activity in the roots of Scots pine seedlings grown at different root temperatures during flushing. J Exp Bot 45:1533–1539. doi:10.1093/jxb/45.11.1533
Sasse JM (2003) Physiological actions of brassinosteroids: an update. J Plant Growth Regul 22:276–288. doi:10.1007/s00344-003-0062-3
Skoczowski A (1999) Influence of cold on selected physiological processes in plants especially on flowering induction. Monograph 8, Institute of Plant Physiology, Polish Academy of Sciences, Krakow, Poland
Skoczowski A, Filek M (1986) Cold induced-changes in lipids from hypocotyls of winter and spring rape. I. The lipid synthesis and fatty acids composition. Acta Physiol Plant 8:203–212
Skoczowski A, Filek M (1994) Changes in fatty acids composition in the subcellular fraction from hypocotyls of winter rape growing at 2°C and 20°C. Plant Sci 98:127–133. doi:10.1016/0168-9452(94)90002-7
Vágújfalvi A, Kerepesi I, Galiba G, Tischner T, Sutka J (1999) Frost hardiness depending on carbohydrate changes during cold acclimation in wheat. Plant Sci 144:85–92. doi:10.1016/S0168-9452(99)00058-8
Vardhini BV, Rao SSR (1998) Effect of brassinosteroids on growth, metabolite content and yield of Arachis hypogaea. Phytochemistry 48:927–930. doi:10.1016/S0031-9422(97)00710-3
Willemot C (1979) Chemical modification of lipids during frost hardening of herbaceous species. In: Lyons JM, Graham D, Raison JK (eds) Low temperature stress in crop plants. Academic, New York, pp 411–430
Yamaryo Y, Kanai D, Awai K, Shimojima M, Masuda T, Shimada H et al (2003) Light and cytokinin play a co-operative role in MGDG synthesis in greening cucumber cotyledons. Plant Cell Physiol 44:844–855. doi:10.1093/pcp/pcg110
Yoshida S, Uemura M (1984) Protein and lipid composition of isolated plasma membranes from orchard grass (Dactylis glomerata L.) and changes during cold acclimation. Plant Physiol 75:31–37
Yoshida S, Uemura M (1990) Responses of the plasma membrane to cold acclimation and freezing stress. In: Larsson C, Moller IM (eds) The plasma membrane. Structure, function, and molecular biology. Springer, Berlin, pp 293–319
Yu JQ, Huang LF, Hu WH, Zhou YH, Mao WH, Ye SF et al (2004) A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J Exp Bot 55:1135–1143. doi:10.1093/jxb/erh124
Zhu B, Ryu SB, Li PH (1990) Effect of abscisic acid biosynthesis inhibitor on cold-induced hardiness in cultured plant cells. Plant Physiol 93(suppl 1):84
Zullo MAT, Kohout L (2004) Semisystematic nomenclature of brassinosteroids. Plant Growth Regul 42:15–28. doi:10.1023/B:GROW.0000014898.30414.33
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Lewak.
Rights and permissions
About this article
Cite this article
Janeczko, A., Hura, K., Skoczowski, A. et al. Temperature-dependent impact of 24-epibrassinolide on the fatty acid composition and sugar content in winter oilseed rape callus. Acta Physiol Plant 31, 71–79 (2009). https://doi.org/10.1007/s11738-008-0202-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-008-0202-2