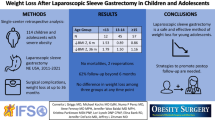
Abstract
Purpose
Despite the rising interest in bariatric surgery (BS) for children and adolescents, algorithms that incorporate BS in weight management (WM) programs are lacking. This study presents the results of the pediatric bariatric surgery clinical pathway employed in our institution.
Materials and Methods
Starting March 2008, we enrolled obese children and adolescents in a standardized multidisciplinary obesity management program. Weight loss, complications, comorbidities, and growth results of those who eventually underwent BS were compared with a matched (age, gender, and height z-score) group of patients on non-surgical WM only.
Results
Up to July 2014, a total of 659 patients received care through the pathway, of whom 291 patients underwent laparoscopic sleeve gastrectomy (LSG). Mean age and pre-LSG body mass index (BMI) were 14.4 ± 4.0 years (range; 5 to 21 years) and 48.3 ± 10.0 (range; 31.8–109.6). Mean BMI change (% excess weight loss) at 1, 2, 3, and 4 postoperative years was −16.9 ± 4.9 (56.6 ± 22.6), −17.5 ± 5.2 (69.8 ± 22.5), −18.9 ± 4.3 (75.1 ± 26.8), and −19.6 ± 6.4 (73.6 ± 24.3), respectively. Postoperatively, complications occurred in 12 patients (4.1 %), with no leaks or mortality, and more than 90 % of comorbidities were resolved or improved without recurrence. Additionally, LSG patients exhibited significantly higher postoperative growth velocity compared to WM patients.
Conclusions
Applying this standardized clinical pathway with its BS component results in safe and successful weight loss for pediatric patients, with low complication rates, maximum comorbidity resolution, and minimum morbidity.
Similar content being viewed by others
Introduction
Obesity affects children and adults across all age groups. More than 30 % of children and adolescents in the USA are currently overweight or obese, whereas in school-age children, the prevalence of obesity as high as 21.4 % [1]. Over the past several decades, a sustained increase in severe forms of obesity has been observed in younger age groups, with current data highlighting that more than 2 % of all children and adolescents in the USA are currently morbidly obese [2]. Unfortunately, purely non-surgical weight management (WM) programs achieve modest results at best [3], raising the demand for an option that can lead to more successful weight loss results, especially for those whose obesity is deemed life-threatening.
In adults, bariatric surgery has been established as a highly effective and safe intervention [4], and with the increasing epidemic of pediatric obesity in most countries, there is a growing interest in bariatric surgery as an effective weight loss option in younger age groups [5–10]. However, concerns about its efficacy, complications, effect on the nutritional status of growing patients, follow-up compliance, timing of surgery, resolution of comorbidities, and possibility of unforeseen complications have made the pediatric community cautious in their approach to bariatric surgery [11]. We believe that many of these concerns can be better addressed by introducing a standardized WM protocol that makes room for bariatric surgery [12–15], with a clinical pathway allowing for reproducible results with regard to safety and efficacy, complications, and other outcome measures including the effect of bariatric surgery on growth.
This study aims to report the pediatric bariatric surgery clinical pathway that was employed for obese children and adolescents who underwent laparoscopic sleeve gastrectomy (LSG) and to compare the results to those of an age- and gender-matched group of non-surgical WM patients who were managed through the same program at our institution. We emphasize on the details of the clinical protocol and the short- and intermediate-term outcomes attained from following this clinical pathway.
Materials and Methods
The clinical pathway described on this paper was employed in our institution beginning in March 2008. Patients who underwent LSG via this protocol until July 2014 were included. In order to study the effect of bariatric surgery on growth, the surgical group was matched for age, gender, and height with obese children and adolescents who are in the non-surgical WM program at the same period.
Weight Management Selection Criteria
The criteria for acceptance into the WM program include an age below 21 years and a body mass index at or greater than the 85th percentile for age. Patients are categorized based on BMI percentile and comorbidity burden and according to internationally accepted standards [16] (Fig. 1).
Weight Management Program Details
A multidisciplinary team (MDT) consisting of a pediatric endocrinologist, a bariatric surgeon, dieticians, nurses, psychologists, and health educators counsels each patient and monitors their progress. Once a month, a full-day workshop is held for new patients and their families, during which the pediatric endocrinologist, the senior dietician, the psychologist, and the health educator, all discuss how to have a healthy lifestyle with parents and children. The discussions include examples of poor choices, substitutions for common unhealthy choices, hands-on training on how to prepare healthy foods, and physical education lessons. Each patient is then regularly seen in the clinic according to their health category (Fig. 1). In the clinic, care is provided via a family-based approach focused on nutrition and behavior counseling. Additionally, patients are prescribed regular physical activity according to their age, and their parents are encouraged to monitor and calculate the duration of physical activity performed each day. Patients are seen monthly by the dietician, the nurse, the psychologist, and the health educator, and every 3 months by the pediatric endocrinologist.
Bariatric Surgery Selection Criteria
Under our care pathway, bariatric surgery is only recommended for children and adolescents who fail WM while their obesity is deemed to have a major impact on their lives. Additionally, it is ensured that the patients and their families are motivated, compliant, have a full understanding of the risks and benefits of the procedure, and are prepared for lifelong commitment.
The criteria include (1) a BMI of at least 40 kg/m2 (or having multiple comorbid conditions with a BMI higher than 35 kg/m2 or above the 99th percentile for age); (2) having undergone the family-based nutritional and behavioral therapy program with our institution’s multidisciplinary team for at least 6 months with failure to achieve weight reduction of at least 10 % from baseline body weight; (3) presence of a dedicated caregiver from the patient’s family; (4) supportive psychological evaluation in the form of behavioral (features of conduct disorder, impulsivity, and aggression), cognitive (psychiatric history, readiness to change, and commitment to instructions), emotional (depression and self-harm including suicide ideation or previous attempt, anxiety, and stress), and psychosocial assessment (activities and interests, friendships, bullying, and social isolation). All evaluations are performed through a one-to-one interview, and patients with significant findings in the interview are further evaluated with the concerned specialist; (5) motivation and realistic expectations by the patient and their family; (6) absence of contraindications for surgery; and (7) informed consent or parental consent with child assent based on patient age.
Preoperative Care
Upon fulfillment of all criteria for bariatric surgery, we evaluate all patients in the multidisciplinary obesity clinic with a specific perioperative protocol. The patients are interviewed 2 weeks prior to admission to assess their readiness and complete understanding of risks and benefits of the surgery. Their investigational workup is reviewed and any questions the patient or their family might have are answered (Tables 1). On this visit, they are provided with an instructions manual regarding the surgery, perioperative care, and postoperative commitments. At the time of the patient’s admission for surgery, nurses are provided with a detailed set of pre-typed preoperative orders for each patient (Table 2). Depending on the hospital’s policies, patients can be admitted to the ward on the day of the surgery or the night before. (See Tables 3 and 4).
Surgical Technique
Patients are positioned in the reverse Trendelenburg French position and a five-trocar approach is used. The abdominal cavity is insufflated with carbon-dioxide to a pressure of 15 mmHg using a 10 mm optic port placed at or within a variable distance above the umbilicus, based on the patient’s age. This port serves as the camera trocar. Four additional trocars (one 12 mm and three 5 mm) are placed under laparoscopic view. The greater curvature is then freed close to the stomach wall, beginning from approximately 2 cm proximal to the pylorus to the angle of His using a Ligasure™ device (Valleylab, USA). The left crus is then dissected and the angle of His is delineated. Posterior adhesions to the pancreas are lysed. A 36-Fr calibrating tube (34-Fr for patients below the age of 12 years) is placed transorally and carefully advanced through the pylorus to the duodenum. At 2–3 cm from the pylorus, the stomach is divided using a linear stapler (Echelon 60 Disposable, Ethicon, Endo-Surgery, Inc., Cincinnati, OH). A green load (4.1 mm) followed by a gold (3.8 mm) and a blue load (3.5 mm) is used for all patients except for those younger than 12 years with thinner stomachs, where only gold and blue loads were used. There is no routine staple line reinforcement or routine testing for leak or drain placement. The resected stomach is then extracted through the 12 mm port site, and the port sites are closed using the Endo Close device (US Surgical TM).
Postoperative Care
As part of the clinical pathway, the attending nurses are provided with a set of pre-typed orders for each patient (Table 2). Scheduled medical visits are performed by the multidisciplinary team over the next one to two postoperative days, providing the patients and their families with specific instructions and guidance as per protocol. The clinical nutritionist provides postoperative diet summary and nutrient supplements to each patient (Tables 3 and 4). Based on preoperative evaluation including clinical and polysomnography results, patients with severe obstructive sleep apnea (OSA) are admitted to the intensive care unit (ICU) overnight. Otherwise, all patients are sent back to a ward staffed with nurses experienced in caring for severely obese patients. On the second postoperative day and upon tolerance of oral intake, patients are discharged with home instructions and long-term follow-up schedule (Table 5).
Outcome Surveillance
All outcomes of interest are prospectively collected in a custom-developed database. It includes clinical research forms (CRF) that capture medical assessment, anthropometric measurements, investigations, and medications prescribed. The CRFs capture data on each of the following stages: (1) Each visit during the non-surgical WM period, (2) preoperative assessment, (3) operative details, (4) in-hospital stay, (5) discharge notes, and (6) postoperative follow-up points (3, 6, and 12 postoperative months and annually thereafter).
Safety of bariatric surgery is comprehensively assessed by documenting all intraoperative and postoperative complications. Additionally, growth after surgery is compared with growth of the non-surgical WM control group through assessing height z-score, which was calculated using the LMS method developed by Cole [17]. This method is a standard technique for constructing age-related growth references and summarizes the distribution of an anthropometric variable at each value of a covariate (age) in terms of three parameters: Box–Cox power (L), median (M), and coefficient of variation (S). After calculating the three parameters at each age from the reference population, these values can be used to calculate z-scores for the respective anthropometric variable. The LMS parameters used in this study were calculated by the Centers for Disease Control and Prevention (CDC) from their growth charts [18]. Growth velocity, which was assessed via measuring height change, was also measured.
Efficacy of bariatric surgery is assessed by measuring adiposity change and resolution of comorbidities. Excess weight lost and BMI change are calculated as previously described [8].
The comorbidities monitored are diabetes, prediabetes, hypertension, prehypertension, dyslipidemia, and sleep apnea, and they are all assessed using internationally accepted definitions that are specific to pediatric age groups [19–22]. Remission and improvement in comorbidities was assessed based on clinical and biochemical parameters with specific cut-off ranges that have been described previously [10].
Results
Patient Characteristics
Between March 2008 and July 2014, 659 pediatric patients were in follow-up with our multidisciplinary clinic. The mean age of patients in the non-surgical WM program (n = 368) was 12.4 ± 4.0 years (range; 5.1–20.9). Eighty-eight (30.2 %) children were aged 5 to 12.99 years, 147 (50.5 %) were aged 13 to 17.99 years, and 56 (19.2 %) were 18 years of age or older. The WM patients had a mean BMI of 38.5 ± 11.3. Patients who eventually underwent bariatric surgery (n = 291) during the study period spent an average of 1.5 ± 0.9 years of follow-up with the multidisciplinary clinic before undergoing surgery, during which their mean BMI change was +0.64 ± 1.1. At the time of surgery, 52 % were female, and their mean (± SD) age and preoperative BMI were 14.4 ± 4.0 years (range; 5–21) and 48.8 ± 11.2 kg/m2, respectively.
Weight Loss
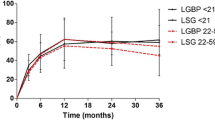
Mean postoperative % excess weight loss (EWL) at 12, 24, 36, and 48 months after surgery was 65.6 ± 22.6 %, 69.8 ± 22.5 %, 75.1 ± 26.8 %, and 73.6 ± 24.30 %, respectively. During this period, the mean BMI change was −16.9 ± 4.9, −17.5 ± 5.2, −18.9 ± 4.3, and −19.6 ± 6.4, respectively. The mean % change in BMI was −34.6 ± 9.6 %, −35.9 ± 10.7 %, −38.7 ± 8.9 %, and −40.2 ± 11.3 % for the follow-up visits (Fig. 2).
Growth
Average preoperative and pre-WM height z-scores were similar (mean preoperative height z-score, −0.09 ± 1.0; mean pre-WM height z-score was −0.10 ± 1.0; p value 0.91). Height z-score of LSG and WM patients was similar at all follow-ups (p value > 0.05). Height change, however, was significantly higher in postoperative patients until the third postoperative year (p value at one, two, three, and four postoperative years; 0.003, 0.001, 0.006, 0.179). Four years after surgery, patients gained an average of 11.2 ± 5.7 cm compared to 8.9 ± 6.4 cm for those who had completed 4 years of non-surgical WM (p value at four postoperative years; 0.179) (Table 6).
Postoperative Complications
Twelve patients (4.1 %) reported complications after LSG, of which six occurred within the first 30 days after surgery. These included two cases of nausea and vomiting with one readmission. Two patients had wound infections at the stomach extracting site (12 mm port). One patient experienced bleeding from the staple line that was managed conservatively with 2 units of packed red blood cells. Another patient was readmitted 2 weeks postoperatively due to a suspected staple line leak with perigastric gas and inflammation. However, further investigations failed to demonstrate an active leak. Consequently, this patient was placed on intravenous antibiotics and total parental nutrition for 2 weeks with complete resolution. Beyond 30 days and within the first 6 months after surgery, two patients were readmitted at separate hospitals with metabolic neuropathy that improved with vitamins B1 and B12 injections. Both patients experienced complete remission of their conditions and were subsequently maintained on a chewable multivitamin formulary that provides 18 mg of vitamin B1, 3 mg of vitamin B6, and 840 mcg of vitamin B12 twice daily (Bariatric Fusion, Elma, NY) with no recurrence of symptoms. Four other patients had postoperative gastroesophageal reflux disease (GERD). There were no reoperations, any major complications, thromboembolic events or mortality. Mean postoperative stay was 3 ± 0.7 days, excluding a 21 years old bedridden male with a baseline weight of 300 kg (BMI 86.7 kg/m2).
Compliance to Follow-up
Follow-up compliance analysis showed that 1163 of 1379 (84.3 %) WM visits were attended on time or after scheduling compared to 1253 of 1333 (94.0 %) LSG patient visits. Overall, the application of the follow-up protocol (rescheduling, contacting patient, and arranging travel assistance) allowed us to obtain 297 WM data points and 265 LSG data points which would have been otherwise lost. Of note, only 12 of the 32 patients (37.5 %) who visited the clinic for their fourth postoperative year visit required application of the protocol, whereas all patients on non-surgical WM had to be contacted and have their visits rescheduled and/or required travel assistance).
Change in Comorbid Conditions
Patients who underwent bariatric surgery experienced substantially higher resolution of comorbidities compared to those on WM, as less than 30 % of the comorbidities suffered by children and adolescents were improved or resolved by the 4 years of WM. Conversely, children and adolescents who underwent LSG experienced resolution of more than 90 % of their comorbidities (Fig. 3).
Discussion
Currently, there is no well-accepted definition of what constitutes a clinical pathway. However, the scientific community has suggested criteria that revolve around the following five points: (1) a multidisciplinary plan of care; (2) translation of guidelines or evidence into local structures; (3) use of plan, pathway, algorithm, guideline, or protocol; (4) have timeframes or criteria-based progression; and (5) standardized care for a specific clinical problem, procedure, or episode of healthcare in a specific population [23]. As pediatric bariatric surgery is currently being performed at very few centers worldwide, we believe that practical guidelines or clinical pathways are essential. There are many concerns in managing children and adolescents undergoing bariatric surgery, and performing these surgical procedures without specific guidelines or clinical pathways will add more complexity to those concerns.
Improvement in outcomes has already been demonstrated with standardizing the surgical care for a wide range of procedures including colorectal surgery [24, 25]. Furthermore, this strategy is believed to reduce treatment costs as care is provided in a more organized manner without squandering hospital resources. This is the case in bariatric surgery, with recently emerging evidence suggesting that the introduction of a fast-track program for Roux-en-y gastric bypass improves short-term recovery and reduces hospital stay and complications [26].
In this study, we highlight several outcomes attained through implementing our clinical pathway. Previously, we reported that the complications in pediatric patients undergoing bariatric surgery are lower than those in adults [6]. Additionally, we demonstrated that the complication rate experienced by both adult and pediatric patients under this clinical pathway is lower than published rates [8]. A review of 15 studies by Shi et al. showed that the mean complication rate following LSG in adults was 11 %, with 0.3 % mortality [27]. Our pediatric patients had a complication rate of 4.1 %, and results from applying this protocol on adult patients have shown mean complication rate of 7 % with no mortality in either age group [6]. Another review published by the American Society for Metabolic and Bariatric Surgery on sleeve gastrectomy reported leak and bleeding rates ranging between 1 and 3 % of patients [28]. Likewise, a systematic review by Gagner and colleagues found that leak rates range between 1.1 and 3.3 % [29]. We attribute the absence of postoperative leak and lower complication rates not only to the operative technique, but also to the specific and highly detailed perioperative clinical pathway employed. In line with published evidence, we observed that having all team members well acquainted with specific guidelines and having them follow a pre-written algorithm leads to minimization of medical errors and maximization of care [30]. Furthermore, it has been found that implementing a standardized care pathway minimizes the learning curve of the surgeon and the team [14, 24].
Among the most debated concerns against bariatric surgery in children and adolescents is whether those procedures affect growth and maturity. Our clinical pathway gave us the opportunity to accurately assess the growth of those who underwent surgery compared to those who only underwent WM. While there was no significant difference in absolute height z-score between the two groups, annual growth velocity was significantly higher in patients who underwent LSG at all postoperative follow-ups. We believe that these findings clearly indicate that LSG has no negative effect on growth of children and adolescents. If anything, LSG may improve the growth of otherwise severely obese children and adolescents, as evident by the significantly higher height gain attained after surgery.
After surgery, patients experienced significant weight loss approaching 20 BMI points at four postoperative years. In contrast to what is observed in adult series, pediatric weight loss in our series was maintained, and patients were more compliant to visits [6, 31]. This could be due to the fact that our healthcare providers are instructed as part of the clinical pathway to educate patients and their families on the importance of adherence to lifelong follow-up, and also assist them in making it to the follow-up appointments through telephone notifications of their upcoming appointments. The ministry of health also offers routine travel assistance for patients outside the clinic city, and our team provides patients with alternative dates for follow-up visits to accommodate missing appointments.
We would like to stress on a few technical points that are included in our clinical pathway and might be a reason for achieving a better surgical outcomes with minimal complications. In our opinion, the safest way to enter the abdomen in morbidly obese children and adolescents is by using direct vision, with a 10–12 mm optic trocar that allows visualization of the abdominal wall layers during entry (Autosuture, Norwalk, CT). This technique requires experience to perform, particularly in identifying each of the layers while using appropriate force. More importantly, knowing when to stop upon entering the peritoneal cavity is critical. The size of the calibrating tube used is dependent on age: for patients below 10–12 years of age, we use a 34-F, and for patients older than 12 years, we use a 36-F tube. We always pass the orogastric tube through the pylorus to the duodenum, as passing it through the pylorus secures its position and allows us to staple along a clear, well demarcated, and fixed route. This also allows us to accurately start stapling at a desired, fixed, and reproducible distance from the pylorus. On the other hand, if the tube is not passed through the pylorus, the distance from the pylorus will be variable in each case due to the tube’s different location in the antrum. The first staple load is fired at a fixed distance of 2 cm from the pylorus. The first staple firings use a green load followed by a gold load due to the increased thickness of the stomach at this level. However, the thickness is less in younger age groups (<12 years) which is why we start with the gold load. The remaining stomach is then divided up to the angle of His using the blue loads and the calibrating tube as a guide. It is very important to flatten the stomach laterally, assuring equal retraction on both anterior and posterior walls of the stomach and not to hug the calibration tube tightly with the stapler. The previously inserted temperature probe and the regular orogastric tube must be removed before firing the loads. Some anesthetists who are not familiar with the surgery protocol might insert the calibrating tube leaving the orogastric tube in place or inadvertently push the temperature probe all the way to the stomach. Additional care should be taken during the first two staple fires to avoid an angle that may narrow the lumen at the incisura, and create a straight staple line avoiding anterior or posterior ‘spiraling’, which can also cause mechanical or functional problems with the sleeve. On one hand, it is important not to leave a significant portion of the fundus that might expand with time and form a pouch leading to food stagnation and possible weight regain. On the other hand, a few millimeters should be left on the side of the gastroesophageal junction during the final staple firing. We do not perform routine staple line reinforcement or routine nasogastric tube or drain placement. Different randomized clinical trials have shown that none of these practices lead to significant differences when used routinely [32]. On the contrary, we believe that over-sewing the staple line might result in a narrow sleeve with higher leak and stricture rates [30]. Before deflating the pneumoperitoneum, an adequate systolic blood pressure must be ensured as low blood pressure may momentarily hide bleeding that becomes evident after a patient’s recovery [33]. We do not perform routine air or blue dye test of the sleeve pouch or a routine radiographic study of the gastrointestinal tract postoperatively, unless there are clinical signs of a leak, which include abnormal temperature or persistent tachycardia. If neither is present on the night of surgery or the first day postoperative, we start the patient on water trial and progress to Stage One (Table 3).
Our clinical pathway serves the purpose of introducing higher efficiency in our practice and helps us to deliver better care to patients and achieve highly successful outcomes. The pathway accommodates standardized non-surgical and surgical weight management methods, preoperative workup, pre-admission procedures, operative procedure, intraoperative, and postoperative care protocols following specific timelines. It is also flexible and has been designed to allow tailoring for each patient’s specific needs, including those with monogenic and syndromic forms of obesity [9].
This study is not without limitations. While this is a review of prospectively collected data, it is still retrospective in nature. Additionally, there was no randomization in allocating the patients into either group, and while this report describes the experience of a single surgeon with this pathway, it would control for inter-surgeon and inter-center confounding variables.
Providing standardized care under a specific clinical pathway will allow different institutions to provide bariatric surgery to children and adolescents. This creates an opportunity for centers to focus on surgical outcomes, particularly the outcomes of children in younger age groups, in whom bariatric surgery is still under debate.
Conclusions
We developed and implemented a new standardized clinical pathway for obese children and adolescents. The results obtained with this pathway give hope for those who fail WM. They suggest that safe and effective outcomes with low complication rates, maximum comorbidity resolution, and reduced morbidity and mortality without impacting growth are all possible by adopting a standardized care plan that accounts for patient assessment, WM protocol, and bariatric procedure technique, perioperative care, and follow-up protocol for children and adolescents.
References
Al-Qahtani A. Surgical approaches to pediatric obesity. In: Ferry Jr RJ, editor. Management of pediatric obesity and diabetes. New York: Springer; 2011. p. 221–48.
Skinner A, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999-2012. JAMA Pediatr. 2014;168(6):561–6.
Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: Public-health crisis, common sense cure. Lancet. 2002;360:473–82.
Chang S, Stoll CT, Song J, Varela J, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149:275–87.
Inge TH, Xanthakos SA, Zeller MH. Bariatric surgery for pediatric extreme obesity: now or later? Int J Obes. 2007;31(1):1–14.
Alqahtani A, Alamri H, Elahmedi M, Mohammed R. Laparoscopic sleeve gastrectomy in adult and pediatric obese patients: a comparative study. Surg Endosc. 2012;26(11):3094–100.
Alqahtani AR. Reply to Letter: "Laparoscopic Sleeve Gastrectomy in 108 Obese Children and Adolescents Aged 5 to 21 Years". Ann Surg. 2013. Epub 2013/08/31.
Alqahtani AR, Antonisamy B, Alamri H, Elahmedi M, Zimmerman VA. Laparoscopic sleeve gastrectomy in 108 obese children and adolescents aged 5 to 21 years. Ann Surg. 2012;256(2):266–73.
Alqahtani AR, Elahmedi M, Alqahtani YA. Bariatric surgery in monogenic and syndromic forms of obesity. Semin Pediatr Surg. 2014;23(1):37–42.
Alqahtani AR, Elahmedi MO, Al Qahtani A. Co-morbidity resolution in morbidly obese children and adolescents undergoing sleeve gastrectomy. Surg Obes Rel Dis. doi:10.1016/j.soard.2014.01.020.
Michalsky M, Reichard K, Inge T, Pratt J, Lenders C. ASMBS pediatric committee best practice guidelines. Surg Obes Relat Dis. 2012;8(1):1–7.
Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008;248(2):189–98.
Main DS, Henderson WG, Pratte K, et al. Relationship of processes and structures of care in general surgery to postoperative outcomes: a descriptive analysis. JACS. 204(6):1157-65.
Lublin M, Lyass S, Lahmann B, Cunneen SA, Khalili TM, Elashoff JD, et al. Leveling the learning curve for laparoscopic bariatric surgery. Surg Endosc. 2005;19(6):845–8.
Birkmeyer JD, Dimick JB, Staiger DO. Operative mortality and procedure volume as predictors of subsequent hospital performance. Ann Surg. 2006;243(3):411–7.
Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128(15):1689–712.
Cole TJ. The LMS, method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44(1):45–60.
Flegal KM, Cole TJ. Construction of LMS parameters for the centers for disease control and prevention 2000 growth charts. National health statistics reports. 2013;63.
Falkner B, Daniels SR. Summary of the fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Hypertension. 2004;44(4):387–8.
Kavey R, Simons-Morton D, de Jesus J. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: full report. 2011 [updated Jan 5, 2011January 5, 2012]; p. S3. 2012.
Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36 Suppl 1:S67-74.
Chervin RD, Weatherly RA, Garetz SL, Ruzicka DL, Giordani BJ, Hodges EK, et al. Pediatric sleep questionnaire: Prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg. 2007;133(3):216–22.
Kinsman L, Rotter T, James E, Snow P, Willis J. What is a clinical pathway? Development of a definition to inform the debate. BMC Med. 2010;8:31.
Senagore A, Duepree H, Delaney C, Brady K, Fazio V. Results of a standardized technique and postoperative care plan for laparoscopic sigmoid colectomy. Dis Colon Rectum. 2003;46(4):503–9.
Lemmens L, van Zelm R, Borel Rinkes I, van Hillegersberg R, Kerkkamp H. Clinical and organizational content of clinical pathways for digestive surgery: a systematic review. Dig Surg. 2009;26(2):91–9.
Dogan K, Kraaij L, Aarts EO, et al. Fast-track bariatric surgery improves perioperative care and logistics compared to conventional care. Obes Surg. 2014. doi:10.1007/s11695-014-1355-2.
Shi X, Karmali S, Sharma AM, Birch DW. A review of laparoscopic sleeve gastrectomy for morbid obesity. Obes Surg. 2010;20(8):1171–7.
ASMBS Clinical Issues Committee. Updated position statement on sleeve gastrectomy as a bariatric procedure. Surg Obes Relat Dis. 2012;8(3):e21–6.
Gagner M, Buchwald JN. Comparison of laparoscopic sleeve gastrectomy leak rates in four staple-line reinforcement options: a systematic review. Surg Obes Relat Dis. 2014. doi:10.1016/j.soard.2014.01.016.
Huerta S, Heber D, Sawicki MP, et al. Reduced length of stay by implementation of a clinical pathway for bariatric surgery in an academic health care center. Am Surg. 2001;67(12):1128–35.
Sieber P, Gass M, Kern B, Peters T, Slawik M, Peterli R. Five-year results of laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2014;10(2):243–9.
Musella M, Milone M, Bellini M, et al. Laparoscopic sleeve gastrectomy. Do we need to oversew the staple line? Ann Ital Chir. 2011;82(4):273–7.
Nguyen NT, Wolfe BM. The physiologic effects of pneumoperitoneum in the morbidly obese. Ann Surg. 2005;241(2):219–26.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no RGP-VPP-186. The authors also acknowledge the contribution from the Shaikh Ali Alshehri Obesity Chair and team members Ms. Nesma M. Mustafa, Layla Alfarra and Nada Abureida. They also thank the participants who took part in the multidisciplinary program.
Conflict of Interest
Dr. Alqahtani and Dr. Elahmedi declare that they have no conflict of interest.
Ethical Clearance
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alqahtani, A.R., Elahmedi, M.O. Pediatric Bariatric Surgery: The Clinical Pathway. OBES SURG 25, 910–921 (2015). https://doi.org/10.1007/s11695-015-1586-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1586-x