Abstract
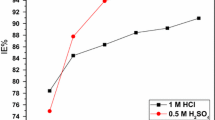
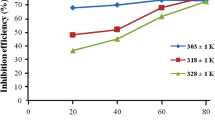
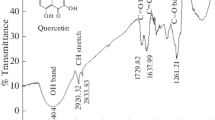
The inhibition effect of 2,3-dihydroxyflavone on the corrosion of mild steel in 100-600 ppm aqueous hydrochloric acid solution has been investigated by weight loss and electrochemical impedance spectroscopy. The corrosion inhibition efficiency increases with increasing concentration and time. The effect of temperature on the corrosion behavior of mild steel in 1 M HCl with addition of inhibitor was studied at the temperature range of 300-330 K. UV-Vis, FTIR, and surface analysis (SEM) was also carried out to establish the corrosion inhibitive property of this inhibitor in HCl solution. The adsorption of this inhibitor on the mild steel surface obeys the Langmuir adsorption isotherm. Electrochemical studies reveal that the inhibitor is a cathodic type.













Similar content being viewed by others
References
S.S. Abd El Rehim, M.A.M. Ibrahim, and K.F. Khalid, The Inhibition of 4-(2′-amino-5′- methylphenylazo) Antipyrine on Corrosion of Mild Steel in HCl Solution, Mater. Chem. Phys., 2001, 70, p 268–273
E.E. Oguzie, C. Unaegbu, C.N. Ogukwe, B.N. Okolue, and A.I. Onuchukwu, Inhibition of Mild Steel Corrosion in Sulphuric Acid Using Indigo Dye and Synergistic Halide Additives, Mater. Chem. Phys., 2004, 84, p 363–368
F. Bentiss, M. Lebrini, and M. Lagrene, Thermodynamic Characterization of Metal Dissolution and Inhibitor Adsorption Processes in Mild Steel/2,5-bis(n-thienyl)-1,3,4-thiadiazoles/Hydrochloric Acid System, Corros. Sci., 2005, 47, p 2915–2931
M. Lebrini, F. Bentiss, H. Vezin, and M. Lagrenée, Inhibitive Properties, Adsorption and a Theoretical Study of 3,5-bis(n-pyridyl)-4-amino-1,2,4-triazoles as Corrosion Inhibitors for Mild Steel in Perchloric Acid, Appl. Surf. Sci., 2005, 252, p 950
S.A. Ali, A.M. El-Shareef, R.F. Al-Ghamdi, and M.T. Saeed, The Isoxazolidines: The Effects of Steric Factor and Hydrophobic Chain Length on the Corrosion Inhibition of Mild Steel in Acidic Medium, Corros. Sci., 2005, 47, p 2659–2678
M.A. Migahed, Electrochemical Investigation of the Corrosion Behaviour of Mild Steel in 2 M HCl Solution in Presence of 1-Dodecyl-4-methoxy pyridinium Bromide, Mater. Chem. Phys., 2005, 93, p 48–53
E.E. Oguzie, Corrosion Inhibition of Aluminium in Acidic and Alkaline Media by Sansevieria trifasciata Extract, Mater. Lett., 2005, 59, p 1076
E.A. Noor, The Inhibition of Mild Steel Corrosion in Phosphoric Acid Solutions by Some N-Heterocyclic Compounds in the Salt Form, Corros. Sci., 2005, 47, p 33–55
A.Y.E. Etre, M. Abdullah, and Z.E.E. Tantawy, Corrosion Inhibition of Some Metals Using Lawsonia Extract, Corros. Sci., 2005, 47, p 385–395
C.A. Loto, A.I. Mohammed, and R.O. Loto, Inhibition Evaluation of Mango Juice Extracts on the Corrosion of Mild Steel in HCl, Corros. Prev. Control, 2003, 50, p 107
S. Rajendran, S. Shanmugapriya, T. Rajalakshmi, and A.J. Amalraj, Corrosion Behaviour of Aluminium in the Presence of an Aqueous Extract of Hibiscus rosasinensis, Corros. Sci., 2005, 61, p 685
M. Hosseini, F.L. Metrtens, M. Ghorbani, and R. Mohammed Arshadi, Asymmetrical Schiff Bases as Inhibitors of Mild Steel Corrosion in Sulphuric Acid Media, Mater. Chem. Phys., 2003, 78, p 800–808
M. Lavanya, D. Kesavan, N. Prabhavathi, and N. Sulochana, Studies on the Inhibitive Effect of 3-Hydroxyflavone on the Acid Corrosion of Mild Steel, Surf. Rev. Lett., 2009, 16, p 845
M.G. Sethuraman and P.B. Raja, Corrosion Inhibition of Mild Steel by Datura Metel, Pigment Resin Technol., 2005, 34, p 327
M.A. Quraishi, I. Ahamad, A.K. Singh, S. Shukla, B. Lal, and V. Singh, N-(Piperidinomethyl)-3-[(pyridylidene) amino]isatin: A New and Effective Acid Corrosion Inhibitor for Mild Steel, Mater. Chem. Phys., 2008, 112, p 1035–1039
P. Kalaiselvi, S. Chellammal, S. Palanichamy, and G. Subramanian, Artemisia pallens as Corrosion Inhibitor for Mild Steel in HCl Medium, Mater. Chem. Phys., 2010, 120, p 643–648
S. K. Rajappa, T. V. Venkatesha, and B. M. Praveen, Chemical treatment of zinc surface and its corrosion inhibition studies, Indian Academy of Sciences, 2008, p 37-41
Y. Abbouda, A. Abourriche, T. Saffaj, M. Berrada, M. Charrouf, A. Bennamara, N.A.L. Himidi, and H. Hannache, A Novel Azo Dye, 8-Quinolinol-5-Azoantipyrine as Corrosion Inhibitor for Mild Steel in Acidic Media, Mater. Chem. Phys., 2005, 105, p 1–5
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gopiraman, M., Sathya, C., Vivekananthan, S. et al. Influence of 2,3-Dihydroxyflavanone on Corrosion Inhibition of Mild Steel in Acidic Medium. J. of Materi Eng and Perform 21, 240–246 (2012). https://doi.org/10.1007/s11665-011-9925-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-011-9925-0