Abstract
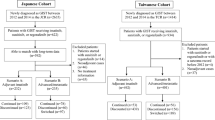
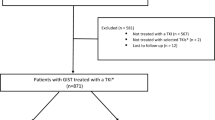
Data about the patterns of care and the specific outcome of elderly patients with advanced gastrointestinal stromal tumors (GISTs) are almost nonexistent. Between 2001 and 2009, 44 patients ≥75 years old with advanced GISTs started first-line imatinib (400 mg/day) in seven participating institutions. Clinical data were collected by reviewing medical records and were entered in a comprehensive database. During the same period, 160 patients with advanced GIST (136 patients <75 years old, 24 patients ≥75 years old) had access to an imatinib blood level testing program. Imatinib plasma concentration (patient dose 400 mg/day) tests were centralized in a single laboratory. Median age was 78 years old (range 75–86). Thirty-six patients (82 %) experienced at least one adverse event (Table 2). Drug-related adverse events were mainly of grades 1 and 2 and were medically manageable. Permanent dose reduction (200–300 mg/day) was required for 20 patients (45.5 %) and was significantly more frequent for patients with performance status (PS) ≥2: 33.5 versus 8.5 %, p = 0.04. Eight patients (18 %) required imatinib interruption for intolerance. Median PFS was 34.4 months (95 % CI 11.5–57.4) (Fig. 1). Median overall survival (OS) was 50.3 months (95 % CI 37–63.5). Performance status <2 was the sole pre-therapeutic factor associated with improved OS. No correlation was found between comorbidities and tolerance or outcome. Imatinib trough plasma concentrations increase with age, although this correlation did not reach statistical significance. First-line imatinib is a feasible and effective treatment in patients with advanced GISTs ≥75 years. Aging seems to have only a moderate impact on imatinib pharmacokinetics. Overall survival is similar to that of younger patients. Comorbidities did not result in increased incidence of toxicity. Careful follow-up regarding tolerance issues should be considered in elderly patients with poor PS.


Similar content being viewed by others
References
Mucciarini C, Rossi G, Bertolini F, Valli R, Cirilli C, Rashid I, Marcheselli L, Luppi G, Federico M (2007) Incidence and clinicopathologic features of gastrointestinal stromal tumors. A population-based study. BMC Cancer 7:230
Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST) (2010 Mar 1) Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol 28(7):1247–53
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Linn BS, Linn MW, Gurel L (1968) Cumulative illness rating scale. J Am Geriatr Soc 16:622–626
Miller MD, Paradis CF, Houck PR et al (1992) Rating chronic medical illness burden in geropsychiatric practice and research: application of cumulative illness rating scale. Psychiatry Res 41:237–248
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Bouchet S, Chauzit E, Ducint D, Castaing N, Canal-Raffin M, Moore N, Titier K, Molimard M (2011) Simultaneous determination of nine tyrosine kinase inhibitors by 96-well solid-phase extraction and ultra performance LC/MS-MS. Clin Chim Acta 412:1060–1067
Titier K, Picard S, Ducint D, Teilhet E, Moore N, Berthaud P, Mahon FX, Molimard M (2005) Quantification of imatinib in human plasma by high-performance liquid chromatography-tandem mass spectrometry. Ther Drug Monit 27:634–640
Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M, Bertulli R, Judson I (2004) Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 364:1127–1134
Latagliata R, Breccia M, Carmosino I, Cannella L, De Cuia R, Diverio D, Frustaci A, Loglisci G, Mancini M, Santopietro M, Stefanizzi C, Volpicelli P, Vozella F, Alimena G (2010) “Real-life” results of front-line treatment with Imatinib in older patients (≥ 65 years) with newly diagnosed chronic myelogenous leukemia. Leuk Res 34:1472–1475
Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, Raymond AK, Bramwell VH, Baker LH, Maki RG, Tanaka M, Hecht JR, Heinrich MC, Fletcher CD, Crowley JJ, Borden EC (2008) Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 26:626–632
Latagliata R, Breccia M, Ferrero D, Cavazzini F, Trawinska MM, Castagnetti F. Imatinib in Very Elderly (> 75 years) CML patients: are low-doses (<400 mg daily) enough? American Society of Hematology Annual Meeting 2011 Abstract 2770
Wedding U, Honecker F, Bokemeyer C, Pientka L, Höffken K (2007) Tolerance to chemotherapy in elderly patients with cancer. Cancer Control 14:44–56
Repetto L, Fratino L, Audisio RA, Venturino A, Gianni W, Vercelli M, Parodi S, Dal Lago D, Gioia F, Monfardini S, Aapro MS, Serraino D, Zagonel V (2002) Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol 20:494–502
Larson RA, Druker BJ, Guilhot F, O'Brien SG, Riviere GJ, Krahnke T, Gathmann I, Wang Y, IRIS (International Randomized Interferon vs STI571) Study Group (2008) Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood 111:4022–4028
Guilhot F, Hughes TP, Cortes J, Druker BJ, Baccarani M, Gathmann I, Hayes M, Granvil C, Wang Y (2012) Plasma exposure of imatinib and its correlation with clinical response in the Tyrosine Kinase Inhibitor Optimization and Selectivity Trial. Haematologica 97:731–738
Demetri GD, Wang Y, Wehrle E, Racine A, Nikolova Z, Blanke CD, Joensuu H, von Mehren M (2009) Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol 27:3141–3147
Nilsson B, Bumming P, Meis-Kindblom JM et al (2005) Gastrointestinal stromal tumours: the incidence, prevalence, clinical course and prognostification in the preimatinib mesylate era—a population-based study in western Sweden. Cancer 103:821–829
Tryggvason G, Gislason HG, Jonasson JG et al (2005) Gastrointestinal stromal tumours in Iceland, 1990–2003: the Icelandic GIST study, a population-based incidence and patholofic risk stratification study. Int J Cancer 117:289–293
DeMatteo RP, Lewis JJ, Brennan MF et al (2000) Two hundred gastrointestinal stromal tumours, recurrence patterns and prognostic factors for survival. Ann Surg 231:51–58
Kam HC, Chun WC, Lin KL et al (2006) Gastrointestinal stromal tumours in a cohort of Chinese patients in Hong Kong. World J Gastroenterol 12:2223–2228
Rubin BP, Heinrich MC, Corless CL (2007) Gastrointestinal stromal tumour. Lancet 369:1731–1741
Tham CK, Poon DY, Li HH, Tan MH, Choo SP, Foo KF (2009) Gastrointestinal stromal tumour in the elderly. Crit Rev Oncol Hematol 70:256–261
Balducci L, Extermann M (2000) Management of cancer in the older person: a practical approach. Oncologist 5:224–237
Balducci L (2006) Management of cancer in the elderly. Oncology 20:135–143
Extermann M, Meyer J (2004) McGinnis M, el al: A comprehensive geriatric intervention detects multiple problems in older breast cancer patients. Crit Rev Oncol Hematol 49:69–75
Girre V, Falcou MC, Gisselbrecht M et al (2008) Does a geriatric oncology consultation modify the cancer treatment plan for elderly patients? J Gerontol A Biol Sci Med Sci 63:724–730
Conflict of interest
The authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Italiano, A., Saada, E., Cioffi, A. et al. Treatment of advanced gastrointestinal stromal tumors in patients over 75 years old: clinical and pharmacological implications. Targ Oncol 8, 295–300 (2013). https://doi.org/10.1007/s11523-012-0243-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-012-0243-8