Abstract
The aim of this paper was to describe the effect of various metal ions on the activity of protocatechuate 3,4-dioxygenase from Stenotrophomonas maltophilia KB2. We also compared activity of different dioxygenases isolated from this strain, in the presence of metal ions, after induction by various aromatic compounds. S. maltophilia KB2 degraded 13 mM 3,4-dihydroxybenzoate, 10 mM benzoic acid and 12 mM phenol within 24 h of incubation. In the presence of dihydroxybenzoate and benzoate, the activity of protocatechuate 3,4-dioxygenase and catechol 1,2-dioxygenase was observed. Although Fe3+, Cu2+, Zn2+, Co2+, Al3+, Cd2+, Ni2+ and Mn2+ ions caused 20–80 % inhibition of protocatechuate 3,4-dioxygenase activity, the above-mentioned metal ions (with the exception of Ni2+) inhibited catechol 1,2-dioxygenase to a lesser extent or even activate the enzyme. Retaining activity of at least one of three dioxygenases from strain KB2 in the presence of metal ions makes it an ideal bacterium for bioremediation of contaminated areas.
Similar content being viewed by others
Introduction
Under aerobic conditions aromatic compounds are usually transformed to a few central intermediates such as catechols, protocatechuates, gentisates and (hydroxy)benzoquinols as a result of introduction of a new hydroxyl group at the ortho- or para-position to the existing one (Lillis et al. 2010; Guzik et al. 2011). Protocatechuic acid is a substrate for protocatechuate 3,4-dioxygenase [EC 1.13.11.3], enzyme catalyzing the intradiol cleavage of the aromatic ring, forming 3-carboxy-cis,cis-muconic acid (Contzen and Stolz 2000; Costas et al. 2004; Guzik et al. 2011). This enzyme belongs to a large class of the nonheme iron-containing dioxygenases and is composed of equimolar amounts of two α and β subunits (Ludwig et al. 1984; Buchan et al. 2000). The crystal structure of this enzyme shows that the high-spin iron(III) is bound to the active site in a distorted trigonal bipyramidal coordination geometry with two inequivalent tyrosine ligands, two histidines, and a hydroxide ion (Valley et al. 2005; Kurahashi et al. 2006; Mayilmurugan et al. 2010). The interaction of a substrate with Fe3+ causes the dissociation of both the axial tyrosine and the hydroxide resulting in a chelated “substrate-Fe3+” complex (Elgren et al. 1997; Vetting et al. 2000). This activates the substrate for an electrophilic attack by dioxygen, which leads to the formation of a peroxo bridge between iron and C4 of substrate (Pau et al. 2005; Borowski and Siegbahn 2006). Next, Criegee rearrangement (acyl migration to the peroxo oxygen) and O–O bond cleavage occurs, leading to the cyclic anhydride formation. The second atom of a molecular oxygen is retained at the Fe3+ as an oxide or hydroxide ion, where it functions as a nucleophile to hydrolyze the anhydride, yielding the ring open product (Vetting et al. 2000; Borowski and Siegbahn 2006). Due to the specific structure and mechanism of protocatechuate 3,4-dioxygenase-catalyzed reaction, the metal ions could influence the activity of this enzyme. During metalloenzymes catalysis, metal ions such as ferrum or calcium, are known to be the activators, since they induce conformational changes in the enzyme to stabilize the bound Fe2+ or to assist the orientation of catalysis site for a substrate binding (Ha et al. 2000). Gopal et al. (2005) suggested that the replacement of the iron in the active site with different metal ions caused the modulation of enzyme activity in accordance with the Irving-Williams order for bivalent metal ions. Several ions are known to be sulfhydryl groups` inhibitors and therefore change the conformation of a protein structure (Ha et al. 2000). In this paper we described the effect of various metal ions (Fe2+, Fe3+, Cu2+, Zn2+, Co2+, Al3+, Cd2+, Ni2+ and Mn2+) on the activity of protocatechuate 3,4-dioxygenase from Stenotrophomonas maltophilia KB2. As in strain KB2 induction of various types of dioxygenases was observed (Guzik et al. 2009), we compared the activity of these enzymes in the presence of different metal ions. Results of our studies seem to be very important for biodegradation processes since the metal ions present in the environment play an important role in bioremediation of aromatic compounds.
Materials and methods
Media and culture conditions for biodegradation of aromatic compounds
Stenotrophomonas maltophilia KB2 (VTT E-113197) is a gram-negative, aromatic compound-degrading bacterium isolated from the activated sludge of the sewage treatment plant in Bytom—Miechowice, Poland (Guzik et al. 2009; Wojcieszyńska et al. 2011b). 250 ml of the sterile MSM (Mineral Salt Medium) supplemented with 1 mM of the tested aromatic compound (phenol, protocatechuic acid, or benzoic acid) were inoculated with KB2 cells to the final optical density of about 0.1 in absorbance scale at λ = 600 nm (OD600), and incubated by shaking at 30 °C for 24 h. While growth of the cultures and complete degradation of the aromatic substrate was observed and OD600 of the culture was above 1.0, the proper volume of the culture was transferred to the new flask with sterile MSM to the final optical density of about 0.1 in absorbance scale at λ = 600 nm (OD600), the successive dose (2 mM and higher) of the aromatic substrate was added/introduced and the cultures were left for incubation for the next 24 h at 30 °C and 125 rpm. The residual aromatic compounds concentration in the culture filtrates was determined by the liquid chromatography.
Induction experiments were carried out in 1-l flasks, containing 500 ml of mineral salts medium and protocatechuic acid or benzoic acid at concentration of 6 and 10 mM, respectively. Protocatechuic acid and benzoic acid were used as the inducers of protocatechuate 3,4-dioxygenase and catechol 1,2-dioxygenase, respectively. Cells in the late exponential growth phase were used for enzymes isolation.
Determination of aromatic compounds concentration
In order to study the degradation of the aromatic compounds, samples were taken periodically from the culture medium and centrifuged (6,000×g, 15 min). Concentration of aromatic compounds in the culture supernatant was determined by HPLC (Merck HITACHI) equipped with a LiChromospher® RP-18 column (4 × 250 mm) and a DAD detector (Merck HITACHI). The wavelength for detection of substrates, composition of eluent and solvent as well as the flow rate were developed separately for each aromatic compound. The mobile phase, in phenol and benzoic acid determination was acetonitryl and water (50:50 v/v), in protocatechuic acid determination was methanol and 1 % acetic acid (25:75 v/v), at the flow rate of 1 ml·min−1. The detection wavelength was set at 285 nm for phenol and at 260 for benzoic and protocatechuic acids. Chemical compounds in the supernatant were identified and quantified by comparing HPLC retention times and UV-visible spectra with external standards.
Preparation of crude enzymatic extract
Cells were harvested in the late exponential growth phase by centrifugation at 5,000×g for 15 min at 4 °C. The cells were then washed with 50 mM phosphate buffer, pH 7.0, and resuspended in the same buffer. The obtained cell extracts were sonicated 6 times for 15 s and centrifuged at 9,500×g for 20 min at 4 °C. The supernatant was used as a crude extract for enzyme assays.
Enzyme assays
Activity of catechol 1,2-dioxygenase [EC 1.13.11.1] was measured spectrophotometrically by the formation of cis,cis-muconic acid at 260 nm (ε260 = 16,800 M−1 cm−1). The reaction mixture contained 20 μl of catechol (50 mM), 67 μl Na2EDTA (20 mM), 893 μl of phosphate buffer pH 7.4 (50 mM) and 20 μl of crude extracts in a total volume of 1 ml. Specific activity of protocatechuate 3,4-dioxygenase was assayed by measuring the consumption of oxygen. The reaction mixture contained 400 μl of protocatechuic acid (10 mM), 2,600 μl of phosphate buffer pH 7.2 (50 mM) and 1,000 μl of crude extract in a total volume of 4 ml according to Hou et al. (1976). Protein concentrations of the crude extracts were determined by the Bradford method (Bradford 1976).
Effect of various metal ions on enzyme’s activity
The effects of metal ions on enzymes` activity were investigated using FeSO4, FeCl3, CuSO4, ZnCl2, CoCl2, AlCl3, CdSO4, NiCl2, and MnSO4. Protocatechuate 3,4-dioxygenase was preincubated in the phosphate buffer (50 mM, pH 7.2) containing: Fe2+, Fe3+, Cu2+, Zn2+, Co2+, Al3+, Cd2+, Ni2+, Mn2+ at a final concentration of 1–3 mM for 3 min at 30 °C. Effect of the metal ions on the activity of catechol 1,2-dioxygenase was studied by incubating it in the presence of above mentioned ions at concentration of 3 mM. After incubation, a residual enzymatic activity was measured as described above.
Results and discussion
Degradation of aromatic compounds by S. maltophilia KB2
Stenotrophomonas maltophilia KB2 is known to degrade a wide spectrum of aromatic compounds (Guzik et al. 2009; Greń et al. 2010; Wojcieszyńska et al. 2011b). In our previous works we found out that in this strain different dioxygenases were induced, depending on the aromatic substrate present in medium. In the presence of protocatechuic acid (3,4-DHB) strain KB2 synthesized protocatechuate 3,4-dioxygenase, while in the presence of benzoic acid (BA) and phenol (PH), activity of catechol 1,2-dioxygenase, and catechol 2,3-dioxygenase, respectively, was observed (Guzik et al. 2009; Wojcieszyńska et al. 2011a). It was interesting to examine the ability of KB2 strain to degrade even high concentrations of above-mentioned substrates: 13 mM of 3,4-DHB, 10 mM of BA, and 12 mM of phenol. As shown in Fig. 1a strain KB2 degraded up to 13 mM 3,4-DHB during 24 h. Significantly lower concentration of this substrate was degraded by Moraxella sp (2 mM) or Burkholderia sp. NCIMB 10467 (1,2 mM) (Sundman 1964; Sterjiades and Pelmont 1989). Additionally, no induction was required for the oxidation of protocatechuate by the latter strain (Sundman 1964; Luo et al. 2008).
Strain KB2 degraded 10 mM BA during 24 h (Fig. 1b) while Streptomyces setonii was capable of degrading only 5 mM of this substrate (An et al. 2000). Moreover, high concentrations of benzoate inhibited growth of Pseudomonas putida (Loh and Chua 2002). In contrast to the results obtained by Loh and Chua (2002), an inhibitory effect of benzoate was not observed by Muthukumar et al. (2009), during degradation of 25 mM benzoic acid by Micrococcus sp.
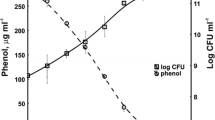
In our work we demonstrated that strain KB2 was also able to utilize 12 mM phenol during 24 h of incubation (Fig. 1c). Similar results were obtained by Shumakova et al. (2009) and Essam et al. (2010), who showed the ability of Rhodococcus opacus strain 1G and Alcaligenes strain TW1 to degrade 10 mM and 12 mM phenol, respectively. Degradation tests for the Acinetobacter strain ATTC11171 made by Adav et al. (2007) showed that this strain fully degraded 5 mM phenol, while at phenol concentrations higher than 5 mM, an inhibitory effect was observed. Phenol at low concentrations (1 and 2 mM) was degraded completely by Ochrobactrum AS1 and Fusarium flocciferum (El-Sayed et al. 2003; Mendonça et al. 2004). Our results show that strain KB2 exhibits ability to degrade a wide range of aromatic compounds at relatively high concentrations what makes it exceedingly attractive for industrial applications especially in bioremediation and wastewater treatment.
Influence of metal ions on protocatechuate 3,4-dioxygenase activity
As it is generally known interactions between metal ions and residues in proteins are important for the protein’s stability, however metals are also known to be powerful inhibitors of the enzyme’s activity. Due to simultaneous contamination of industrial wastes by aromatic compounds and metals (Wu et al. 2008; Deeb and Altalhi 2009), there is an increasing interest in identification of enzymes that degrade aromatic structure and are resistant, among other factors, to the metal ions. In our study we examined influence of various metal ions on protocatechuate 3,4-dioxygenase, the non-heme iron—containing enzyme that catalyzes the ortho cleavage of the aromatic ring between the vicinal hydroxyls to form β-carboxy-cis,cis-muconic acid (Luo et al. 2008).
Addition of Zn2+, Co2+, Al3+, Cd2+ and Ni2+ ions caused a 20–80 % reduction of an initial protocatechuate 3,4-dioxygenase activity (Table 1). The presence of 1 and 2 mM of Mn2+ had no effect on the examined enzyme’s activity which could be explained by low toxicity of this ion (Nies 2000). About 70 % inhibition of the enzyme’s activity was observed after the addition of Cu2+ to the mixture reaction (Table 1). Inhibition of protocatechuate 3,4-dioxygenase caused by various metals was observed also by other authors (Nozaki et al. 1968; Hou et al. 1976; Iwagami et al. 2000). Metal ions can lead to the conformational changes in the enzymes such as a reduction in α-helices and β sheets, which can result in the loss of enzymatic activity (Latha et al. 2011). On the other hand, Gopal et al. (2005) observed no influence of Cu2+on the enzymatic activity of iron-containing quercetin 2,3-dioxygenase. They suggested that replacement of the iron at the active site of the enzyme with other metal may modulate its activity in accordance with the Irving-Williams studies on the stability of the metal complexes (Gopal et al. 2005; Matera et al. 2008). Obtained results allow us to assume that stability of the metal-enzyme complex formed in the active site of our enzyme was insufficient for its activity. Additionally, binding of transition metals ions such as Fe3+, Cu2+, Zn2+, Co2+, Cd2+, Ni2+, Mn2+ to the thiol groups of protocatechuate 3,4-dioxygenase might deactivate the enzyme. Moreover, protocatechuic acid, a substrate for protocatechuate 3,4-dioxygenase may form chelates with metals and these complexes are less accessible for the enzyme.
Intradiol dioxygenases contain a trivalent metal ion that is coordinated by four strictly conserved amino acid residues and one solvent molecule (Valley et al. 2005; Matera et al. 2008). A typical characteristic of most ortho-fission dioxygenases described so far is the increase of its activity by the addition of Fe3+ (Yeom and Yoo 1997; Iwagami et al. 2000). Iwagami et al. (2000) and Wu et al. (2008) stated that in metal ion catalysis, metals act as activators which induce conformational changes of the enzyme to stabilize it or support substrate binding by providing correct orientation of the catalytic site. However, we did not observe this effect. As shown in Table 1, the protocatechuate 3,4-dioxygenase from strain KB2 was inhibited by the addition of Fe3+. Similar effect was observed by Yeom and Yoo (1997) for Alcaligenes xylosoxidans catechol 1,2-dioxygenase. Transition metals such as Fe3+ rapidly react with sulfhydryl groups of cysteine residues which in turn affect tertiary structure of the enzyme. Partial loss of protocatechuate 3,4-dioxygenase’s activity in the presence of ferric ions might be caused by binding of Fe3+ ion to a site other than the active site of enzyme resulting in conformational changes in protein structure. To our surprise, the Fe2+ ions activated the examined enzyme. Similar effect was also observed by Yeom and Yoo (1997) in studies on the influence of metal ions on catechol 1,2-dioxygenases from A. xylosoxidans Y234. The influence of Fe2+ on protocatechuate 3,4-dioxygenase remains unsolved and needs further investigations.
Effects of various metal ions on dioxygenases’ activity
Stenotrophomonas maltophilia KB2 synthesizes three types of dioxygenases depending on the aromatic substrate used (Guzik et al. 2009). Two of them (catechol 1,2-dioxygenase and protocatechuate 3,4-dioxygenase) belong to the intradiol dioxygenases family composed of enzymes with trivalent iron coordinated by one molecule of water, two histidine and two tyrosine residues, and their active sites are structurally very similar. In contrast catechol 2,3-dioxygenase from this strain is a member of extradiol dioxygenases family which comprises enzymes with bivalent iron in the active site (Vetting et al. 2000; Guzik et al. 2011; Wojcieszyńska et al. 2011b). The ability of strain KB2 to induce three types of dioxygenases makes it a very useful tool in bioremediation processes. Since contamination of the environment with aromatic compounds only is extremely rare it was very interesting to study the influence of metals at 3 mM concentration on the activity of dioxygenases isolated from this strain. Our previous studies on catechol 2,3-dioxygenase from strain KB2 shown activation of this enzyme in the presence of Zn2+ while Fe2+, Fe3+, Co2+, Cu2+, Al3+, Cd2+, Ni2+, Mn2+ reduced its activity (Guzik et al. 2010). Ni2+ and Cu2+ caused almost total loss of catechol 2,3-dioxygenase activity. Contrary to catechol 2,3-dioxygenase, almost all metal ions used in this study had no negative effects on catechol 1,2-dioxygenase from strain KB2 (Table 2). Similar results were obtained by Tsai and Li (2007) for Fe2+, Co2+, Cu2+ and Mn2+ions. Nevertheless, an inhibition of catechol 1,2-dioxygenase by metal ions was often observed (Hou et al. 1976; Yeom and Yoo 1997; Wang et al. 2006). Surprisingly, Cd2+ ions, which are known to be extremely toxic, caused high activation of catechol 1,2-dioxygenase (Table 2). The same effect was observed by Tokheim et al. (2005) for aryl sulfatase. Resistance of catechol 1,2-dioxygenase to Cd2+ seems to be very important, as these ions are frequent pollutants in aromatic hydrocarbons contaminated sites (Tsai et al. 2009; Stingu et al. 2012).
The complete inhibition of catechol 1,2-dioxygenase activity was observed in the presence of Ni2+. As it is known this cation tends to bind to cysteine or histidine residues in the proteins (Nies 1999). Since histidines are the key residues forming the active site of the intradiol dioxygenases, the loss of catechol 1,2-dioxygenase activity was probably connected with interaction of Ni2+ with this amino acid. In spite of lower or even no activity of both: catechol 1,2- and 2,3-dioxygenase in the presence of nickel ions, strain KB2 could be still used in degradation of aromatic compound under these conditions due to the fact that protocatechuate 3,4-dioxygenase from this strain was minimally affected by Ni2+ (Table 2).
In summary, degradation of the selected aromatic compounds by S. maltophilia KB2 is catalyzed by one of three types of dioxygenases induced in this strain depending on the substrate used. These enzymes showed different sensitivity to the metal ions. Catechol 1,2- and 2,3-dioxygenase from this strain were strongly inhibited by Ni2+ ions while under the same conditions, protocatechutae 3,4-dioxygenase retained 80 % of its initial activity. In contrast Cu2+ ions inhibited protocatechuate 3,4-dioxygenase and catechol 2,3-dioxygenase while any negative effect of these ions on catechol 1,2-dioxygenase was observed. Induction of three types of dioxygenases in S. maltophilia KB2 ensure a degradation of aromatics which are present in the environment simultaneously contaminated with various metal ions.
References
Adav SS, Chen M-Y, Lee D-J, Ren N-Q (2007) Degradation of phenol by Acinetobacter strain isolated from aerobic granules. Chemosphere 67:1566–1572
An H-R, Park H-J, Kim E-S (2000) Characterization of benzoate degradation via ortho-cleavage by Streptomyces setonii. J Microbiol Biotechnol 10(1):111–114
Borowski T, Siegbahn EM (2006) Mechanism of catechol ring cleavage by non-heme iron intradiol dioxygenases: a hybrid DFT study. J Am Chem Soc 128:12941–12953
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 7:248–254
Buchan A, Collier LS, Neidle EL, Moran MA (2000) Key aromatic-ring-cleaving enzyme, protocatechuate 3,4-dioxygenase, in the ecologically important marine Roseobacter lineage. Appl Environ Microbiol 66(11):4662–4672
Contzen M, Stolz A (2000) Characterization of the genes for two protocatechuate 3,4-dioxygenases from the 4-sulfocatechol-degrading bacterium Agrobacterium radiobacter strain S2. J Bacteriol 182(21):6123–6129
Costas M, Mehn MP, Jensen MP, Que L (2004) Dioxygen activation at mononuclear nonheme iron active sites: enzymes, models, and intermediates. Chem Rev 104:939–986
Deeb BE, Altalhi AD (2009) Degradative plasmid and heave metal resistance plasmid naturally coexist in phenol and cyanide assimilating bacteria. Am J Biochem Biotechnol 5(2):84–93
Elgren TE, Orville AM, Kelly KA, Lipscomb JD, Ohlendorf DH, Que L (1997) Crystal structure and resonance Raman studies of protocatechuate 3,4-dioxygenase complexed with 3,4-dihydroxyphenylacetate. Biochemistry 36:11504–11513
El-Sayed WE, Ibrahim MK, Abu-Shady M, El-Beih F, Ohmura N, Saiki H, Ando A (2003) Isolation and identification of a novel strain of the genus Ochrobactrum with phenol-degrading activity. J Biosci Bioeng 96(3):310–312
Essam T, Amin MA, Tayeb OE, Mattiasson B, Guieysse B (2010) Kinetics and metabolic versality of highly tolerant phenol degrading Alcaligenes strain TW1. J Hazard Mater 173:783–788
Gopal B, Madan LL, Betz SF, Kossiakoff AA (2005) The crystal structure of a quercetin 2,3-dioxygenase from Bacillus subtilis suggests modulation of enzyme activity by a change in the metal ion at the active site(s). Biochemistry 44:193–201
Greń I, Wojcieszyńska D, Guzik U, Perkosz M, Hupert-Kocurek K (2010) Enhanced biotransformation of mononitrophenols by Stenotrophomonas maltophilia KB2 in the presence of aromatic compounds of plant origin. World J Microbiol Biotechnol 26:289–295
Guzik U, Greń I, Wojcieszyńska D, Łabużek S (2009) Isolation and characterization of a novel strain of Stenotrophomonas maltophilia possessing various dioxygenases for monocyclic hydrocarbon degradation. Braz J Microbiol 40:285–291
Guzik U, Wojcieszyńska D, Greń I, Hupert-Kocurek K (2010) Activity of catechol dioxygenases In the presence of some heavy metal ions: bioremediation of environment polluted with aromatic compounds. Ochrona Srodowiska 32(1):9–13 (in Polish)
Guzik U, Greń I, Hupert-Kocurek K, Wojcieszyńska D (2011) Catechol 1,2-dioxygenase from the new aromatic compounds-degrading Pseudomonas putida strain N6. Int Biodeterior Biodegrad 65:504–512
Ha YM, Jung YH, Kwon DY, Kim Y, Kim C-K, Min KH (2000) Reaction characteristics of 4-methylcatechol 2,3-dioxygenase from Pseudomonas putida SU10. J Microbiol Biotechnol 10(1):35–42
Hou ChT, Lillard MO, Schwartz RD (1976) Protocatechuate 3,4-dioxygenase from Acinetobacter calcoaceticus. Biochemistry 15(3):582–588
Iwagami SG, Yang K, Davies J (2000) Characterization of the protocatechuic acid catabolic gene luster from Streptomyces sp. strain 2065. Appl Environ Microbiol 66(4):1499–1508
Kurahashi T, Oda K, Sugimoto M, Ogura T, Fujii H (2006) Trigonal-bipyramidal geometry induced by an external water ligand in a sterically hindered iron salen complex, related to the active site of protocatechuate 3,4-dioxygenase. Inorg Chem 45(19):7709–77021
Latha R, Mandappa IM, Thakur MS, Manonmani HK (2011) Influence of metal ions on dehydrohalogenase activity. Afr J Basic Appl Sci 3(2):45–51
Lillis L, Clipson N, Doyle E (2010) Quantification of catechol dioxygenase gene expression in soil during degradation of 2,4-dichlorophenol. FEMS Microbiol Ecol 73:363–369
Loh K-C, Chua S-S (2002) Ortho pathway of benzoate degradation in Pseudomonas putida: induction of meta pathway at high substrate concentration. Enzyme Microb Technol 30:620–626
Ludwig ML, Weber LD, Ballou DP (1984) Characterization of crystals of protocatechuate 3,4-dioxygenase from Pseudomonas cepacia. J Biol Chem 259(23):14840–14842
Luo S, Zhang J-J, Zhou N-Y (2008) Molecular cloning and biochemical characterization of protocatechuate 3,4-dioxygenase in Burkholderia sp. NCIMB 10467. Microbiology 35(5):712–719
Matera I, Ferraroni M, Bürger S, Scozzafava A, Stolz A, Briganti F (2008) Salicylate 1,2-dioxygenase from Pseudoaminobacter salicylatoxidans: crystal structure of a pelicular ring-cleaving dioxygenase. J Mol Biol 380:856–868
Mayilmurugan R, Sankaralingam M, Suresh E, Palaniandavar M (2010) Novel square pyramidal iron(III) coplex of linear tetradentate bis(phenolate) ligands as structural and reactive models dor intradiol-cleaving 3,4-PCD enzymes: quinine formation vs. intradiol cleavage. Dalton Trans 39:9611–9625
Mendonça E, Martins A, Anselmo M (2004) Biodegradation of natural phenolic compounds as a single and mixed substrates by Fusarium flocciferum. Electron J Biotechnol 7(1):30–37
Muthukumar K, Bharath Ch, Pugalenthi V, Velan M (2009) Biodegradation kinetics of benzoic and anthranilic acids by Micrococcus sp. J Sci Ind Res 68:900–903
Nies DH (1999) Microbial heavy-metal resistance. Appl Microbiol Biotechnol 51:730–750
Nies DH (2000) Heavy metal-resistant bacteria as extremophiles: molecular physiology and biotechnological use of Ralstonia sp. CH34. Extremophiles 4:77–82
Nozaki M, Ono K, Nakazawa T, Kotani S, Hayaishi O (1968) Metapyrocatechase. The role of iron and sulfhydryl groups. J Biol Chem 243(10):2682–2690
Pau MYM, Davis MI, Orville AM, Lipscomb JD, Solomon EI (2005) Spectroscopic and electronic structure study of the enzyme-substrate complex of intradiol dioxygenases: substrate activation by a high-spin ferric non-heme iron site. J Am Chem Soc 129:1944–1958
Shumakova ES, Solyanikova IP, Plotnikova EG, Golovleva LA (2009) Phenol degradation by Rhodococcus opacus strain 1G. Appl Biochem Microbiol 45(1):43–49
Sterjiades R, Pelmont J (1989) Occurrence of two different forms of protocatechuate 3,4-dioxygenase in a Moraxella sp. Appl Environ Microbiol 55(2):340–347
Stingu A, Volf I, Popa VI, Gostin I (2012) New approaches concerning the utilization of natural amendments in cadmium phytoremediation. J Crop Prod 35:53–60
Sundman V (1964) A description of some lignanolytic soil bacteria and their ability to oxidize simple phenolic compounds. J Gen Microbiol 36:171–183
Tokheim AM, Spannaus-Martin DJ, Martin BL (2005) Evidence for the Cd2+ activation of the aryl sulfatase from helix pomatia. Biometals 18:537–540
Tsai S-Ch, Li Y-K (2007) Purification and characterization of a catechol 1,2-dioxygenase from a phenol degrading Candida albicans TL3. Arch Microbiol 187:199–206
Tsai ChJ, Chen ML, Chang KF, Chang FK, Mao IF (2009) The pollution characteristics of odor, volatile organochlorinated compounds and polycyclic aromatic hydrocarbons emitted from plastic waste recycling plants. Chemosphere 74:1104–1110
Valley MP, Brown CK, Burk DL, Vetting MW, Ohlendorf DH, Lipscomb JD (2005) Roles of the equatorial tyrosyl iron ligand of protocatechuate 3,4-dioxygenase in catalysis. Biochemistry 44:11024–11039
Vetting MW, D’Argenio DA, Ornston LN, Ohlendorf DH (2000) Structure of Acinetobacter strain ADP1 protocatechuate 3,4-dioxygenase at 2.2 Å resolution: implication for the mechanism of an intradiol dioxygenase. Biochemistry 39:7943–7955
Wang ChL, You SL, Wang S-L (2006) Purification and characterization of a novel catechol 1,2-dioxygenase from Pseudomonas aeruginosa with benzoic acid as a carbon source. Process Biochem 41:1594–1601
Wojcieszyńska D, Guzik U, Greń I, Perkosz M, Hupert-Kocurek K (2011a) Induction of aromatic ring—cleavage dioxygenases in Stenotrophomonas maltophilia strain KB2 in cometabolic systems. World J Microbiol Biotechnol 27:805–811
Wojcieszyńska D, Hupert-Kocurek K, Greń I, Guzik U (2011b) High activity catechol 2,3-dioxygenase from the cresols-degrading Stenotrophomonas maltophilia strain KB2. Int Biodeterior Biodegrad 65:853–858
Wu L-Z, Ma B-L, Zou D-W, Tie Z-X, Wang J, Wang W (2008) Influence of metal ions on folding pathway and conformational stability of bovine serum albumin. J Mol Struct 877:44–49
Yeom SH, Yoo YJ (1997) Overcoming the inhibition effects of metal ions in the degradation of benzene and toluene by Alcaligenes xylosoxidans Y234. Korean J Chem Eng 14(3):204–208
Acknowledgments
This work was supported by Polish Ministry of Science and Higher Education (IP2010012170). Małgorzata Sitnik is acknowledged for an excellent technical assistance.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Guzik, U., Hupert-Kocurek, K., Sałek, K. et al. Influence of metal ions on bioremediation activity of protocatechuate 3,4-dioxygenase from Stenotrophomonas maltophilia KB2. World J Microbiol Biotechnol 29, 267–273 (2013). https://doi.org/10.1007/s11274-012-1178-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-012-1178-z