Abstract
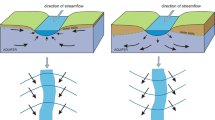
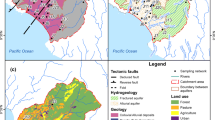
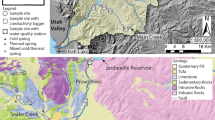
Filtered total mercury (FTHg) concentrations in a rapidly urbanizing area ranged from 50 to 250 ng/L in surface waters of the Squankum Branch, a tributary to a major river (Great Egg Harbor River (GEHR)) traversing both urban and forested/wetland areas in the Coastal Plain of New Jersey. An unsewered residential area with Hg-contaminated well water (one of many in the region) is adjacent to the stream’s left bank. Although the region’s groundwater contains total Hg (THg) at background levels of <10 ng/L, water from about 700 domestic wells in urbanized areas completed in the acidic, quartzose unconfined aquifer typically at depths 20 to 30 m below land surface has been found to exceed 2,000 ng/L (the USEPA maximum contaminant level). Within urbanized areas, THg concentrations in shallow groundwater (<20 m below land surface at or near the water table) and the potential for Hg transport were not well known, representing a considerable knowledge gap. Sampling of streamwater in, and groundwater discharge to, the Squankum Branch watershed revealed that concentrations of THg generally were in the range of 1 to 10 ng/L, but narrow plumes (“plumelets”) of shallow groundwater discharging to the stream from the opposing banks contained FTHg at a concentration > 5,000 ng/L (left bank) and nearly 2,000 ng/L (right bank). The Hg content of bankside soils and sediments was high (up to 12 mg/kg) and mostly acid leachable where groundwater with high Hg concentrations discharged, indicating contributions of Hg by both runoff and shallow groundwater. Elevated concentrations of nutrients and chloride in some groundwater plumelets likely indicated inputs from septic-system effluent and (or) fertilizer applications. The Hg probably derives mainly from mercurial pesticide applications to the former agricultural land being urbanized. The study results show that soil disturbance and introduction of anthropogenic substances can mobilize Hg from soils to shallow groundwater and the Hg contamination travels in narrow plumelets to discharge points such as stream tributaries. In the entire GEHR watershed, THg concentrations in groundwater discharging to streams in urban areas tended to be higher than concentrations in water discharging to streams of forested areas, consistent with the results from this small watershed. Other areas with similar quartzose coastal aquifers, land-use history, and hydrogeology may be similarly vulnerable to Hg contamination of shallow groundwater and associated surface water.







Similar content being viewed by others
References
Aastrup, M., Johnson, J., Bringmark, E., Bringmark, I., & Iverfeldt, Å. (1991). Occurrence and transport of mercury within a small catchment area. Water, Air, and Soil Pollution, 56, 155–167.
Arsenholt-Bindslev, D., & Larsen, A. H. (1996). Mercury levels and discharge in wastewater from dental clinics. Water, Air, and Soil Pollution, 86, 93–99.
Ayers, M.A., Kennen, J.G., & Stackelberg, P.E. (2000). Water quality in the Long Island-New Jersey Coastal drainages, New York and New Jersey, 1996–98. US Geological Survey Circular 1201.
Babiarz, C. L., Hurley, J. P., Benoit, J. M., Shafer, M. M., Andren, A. W., & Webb, D. A. (1998). Seasonal influences on partitioning and transport of total and methylmercury in rivers from contrasting watersheds. Biogeochemistry, 41, 237–257.
BAE Systems (2003). BAE systems ADR, Digital color infrared (CIR) orthophotography of New Jersey. Trenton: New Jersey Office of Information Technology.
Barringer, J.L., MacLeod, C.L., & Gallagher, R.A. (1997). Mercury in ground water, soils, and sediments of the Kirkwood—Cohansey aquifer system in the New Jersey Coastal Plain. US Geological Survey Open-File Report 95-475.
Barringer, J.L., Szabo, Z., & Barringer, T.H. (1998). Arsenic and metals in soils in the vicinity of the Imperial Oil Company Superfund site, Marlboro Township, Monmouth County, New Jersey. U.S Geological Survey Water-Resources Investigations Report 98–4016.
Barringer, J.L., & MacLeod, C.L. (2001). Relation of mercury to other chemical constituents in ground water in the Kirkwood-Cohansey aquifer system, New Jersey Coastal Plain, and mechanisms for mobilization of mercury from sediments to ground water. US Geological Survey Water-Resources Investigations Report 95-4230.
Barringer, J. L., Szabo, Z., Kauffman, L. J., Barringer, T. H., Stackelberg, P. E., Ivahnenko, T., et al. (2005). Mercury concentrations in water from an unconfined aquifer system, New Jersey Coastal Plain. Science of the Total Environment, 346, 169–183.
Barringer, J. L., Szabo, Z., Schneider, D., Atkinson, W. D., & Gallagher, R. A. (2006). Mercury in ground water, septage, leach-field effluent, and soils in residential areas, New Jersey coastal plain. Science of the Total Environment, 361, 144–162.
Barringer, J. L., & Szabo, Z. (2006). Overview of investigations into mercury in ground water, soils, and septage, New Jersey Coastal Plain. Water, Air, and Soil Pollution, 175, 193–221.
Barringer, J. L., Riskin, M. L., Szabo, Z., Reilly, P. A., Rosman, R., Bonin, J. L., et al. (2010). Mercury and methylmercury dynamics in a Coastal Plain watershed, New Jersey, USA. Water, Air, and Soil Pollution, 212, 251–273.
Bodaly, R. A., Rudd, J. W. M., & Flett, R. J. (1998). Effect of urban sewage treatment on total and methyl mercury concentrations in effluents. Biogeochemistry, 40, 279–291.
Bollen, A., Wenke, A., & Biester, H. (2008). Mercury speciation analyses in HgCl2-contaminated soils and groundwater—Implications for risk assessment and remediation strategies. Water Research, 42, 991–1000.
Bradley, P. & Journey, C. (2012) Hydrology and methylmercury availability in Coastal Plain streams; chapter 8, pp. 169–190. In: Nayak, P, (ed.) Water resources management and modeling. InTech, Open Access Publishing. Available at: http//www.intechopen.com/books/water-resources-management-and-modeling.
Bradley, P. M., Journey, C. A., Chapelle, F. H., Lowery, M. A., & Conrad, P. A. (2010). Flood hydrology and methylmercury availability in Coastal Plain rivers. Environmental Science and Technology, 44, 9285–9290.
Bradley, P. M., Journey, C. A., Lowery, M. A., Brigham, M. E., Burns, D. A., Button, D. T., et al. (2012). Shallow groundwater mercury supply in a Coastal Plain stream. Environmental Science and Technology, 46, 7503–7511.
Brumbaugh, W.G., Krabbenhoft, D.P., Helsel, D.R., Weiner, J.G., & Echols, K.R. (2001). A national pilot study of mercury contamination of aquatic ecosystems along multiple gradients: bioaccumulation in fish. US Geological Survey Biological Science Report 2001-0009.
Canário, J., Vale, C., & Nogueira, M. (2008). The pathway of mercury in contaminated waters determined by association with organic carbon (Tagus Estuary, Portugal). Applied Geochemistry, 23, 519–528.
Carter, R.W., & Davidian, J. (1968). General procedure for gaging streams. US Geological Survey Techniques of Water Resources Investigations, bk 3, chap A6.
Chapelle, F. H., Bradley, P. M., McMahon, P. B., Kaiser, K., & Benner, R. (2011). Dissolved oxygen as an indicator of bioavailable dissolved organic carbon in groundwater. Ground Water, 50, 230–241. doi:10.1111/j.1745-6584.2011.00835.x.
Clarkson, T., Magos, L., & Myers, G. (2003). The toxicology of mercury—current exposures and clinical manifestations. The New England Journal of Medicine, 349, 1721–1737.
Clesceri, L. S., Greenberg, A. E., & Eaton, A. D. (Eds.). (1998). Method 4500-52—sulfide. Standard methods for analysis of water and wastewater (20th ed.). Denver: American Public Health Association.
Cline, J. D. (1969). Spectrophotometric determination of hydrogen sulfide in natural waters. Limnology and Oceanography, 11, 454–458.
Datry, T., Malard, F., & Gibert, J. (2004). Dynamics of solutes and dissolved oxygen in shallow urban groundwater below a stormwater infiltration basin. Science of the Total Environment, 329, 215–229.
El-Farhan, Y. H., Denovio, N. M., Herman, J. S., & Hornberger, G. M. (2000). Mobilization and transport of soil particles during infiltration experiments in an agricultural field, Shenandoh Valley, Virginia. Environmental Science and Technology, 34, 3555–3559.
Guentzel, J. L. (2009). Wetland influences on mercury transport and bioaccumulation in South Carolina. Science of the Total Environment, 407, 1344–1353.
Grigal, D. F. (2003). Mercury sequestration in forests and peatlands: a review. Journal of Environmental Quality, 32, 393–405.
Haitzer, M., Aiken, G. R., & Ryan, J. N. (2002). Binding of mercury(II) to dissolved organic matter: the role of the mercury-to-DOM concentration ratio. Environmental Science and Technology, 36, 3564–3570.
Haitzer, M., Aiken, G. R., & Ryan, J. N. (2003). Binding of mercury(II) to aquatic humic substances: influence of pH and source of humic substances. Environmental Science and Technology, 37, 2436–2441.
Hissler, C., & Probst, J. L. (2006). Chlor-alkali industrial contamination and riverine transport of mercury: distribution and partitioning of mercury between water, suspended matter, and bottom sediment of the Thur River, France. Applied Geochemistry, 21, 1837–54.
Hjortenkrans, D. S. T., Berback, B. G., & Häggerud, A. V. (2007). Metal emissions from brake linings and tires: case studies of Stockholm, Sweden 1995/1998 and 2005. Environmental Science and Technology, 41, 5224–5230.
Hsu, H., & Sedlak, D. L. (2003). Strong Hg (II) complexation in municipal wastewater effluent and surface waters. Environmental Science and Technology, 37, 2743–2749.
Hurley, J. P., Benoit, J. M., Babiarz, C. L., Shafer, M. M., Andren, A. W., Sullivan, J. R., et al. (1995). Influences of watershed characteristics on mercury levels in Wisconsin rivers. Environmental Science and Technology, 29(7), 1867–1875.
Kaplan, D. I., Bertsch, P. M., Adriano, D. C., & Miller, W. P. (1993). Soil-borne colloids as influenced by water flow and organic carbon. Environmental Science and Technology, 27, 1193–2000.
Kauffman, L.J., Baehr, A.L., Ayers, M.A., & Stackelberg, P.E. (2001). Effects of land use and travel time on the distribution of nitrate in the Kirkwood–Cohansey aquifer system in southern New Jersey. US Geological Survey Water-Resources Investigations Report 01-4117.
Koterba, M.T., Andres, A.S.,Vrabel, J., Crilley, D.M., Szabo, Z., DeWild, J.T., et al. (2006). Occurrence and distribution of mercury in the surficial aquifer, Long Neck peninsula, Sussex County, Delaware, 2003–04. US Geological Survey Scientific Investigations Report 2006-5011.
Krabbenhoft, D. P., Benoit, J. M., Babiarz, C. L., Hurley, J. P., & Andren, A. W. (1995). Mercury cycling in the Allequash Creek watershed, northern Wisconsin. Water, Air, and Soil Pollution, 80, 425–433.
Lin, C.-C., Chen, S.-J., Huang, K.-L., Hwang, W.-I., Chang-Chien, G.-P., & Lin, W.-Y. (2005). Characteristics of metals in nano/ultrafine/coarse particles collected beside a heavily trafficked road. Environmental Science and Technology, 39, 8113–8122.
Lough, G. C., Schauer, J. J., Park, J.-S., Shafer, M. M., Deminter, J. T., & Weinstein, J. P. (2005). Emissions of metals associated with motor vehicle roadways. Environmental Science and Technology, 39, 826–836.
Lyons, W. B., Fitzgibbon, T. O., Welch, K. A., & Carey, A. E. (2006). Mercury geochemistry of the Scioto River, Ohio: impact of agriculture and urbanization. Applied Geochemistry, 21, 1880–1888.
Mason, R. P., & Sullivan, K. A. (1998). Mercury and methylmercury transport through an urban watershed. Water Research, 32(2), 321–330.
Modica, E., Buxton, H. T., & Plummer, L. N. (1998). Evaluating the source area and residence times of ground-water seepage to streams, New Jersey Coastal Plain. Water Resources Research, 34, 2797–2810.
Murphy, E., Dooley, J., Windom, H. L., & Smith, R. G., Jr. (1994). Mercury species in potable ground water in southern New Jersey. Water, Air, and Soil Pollution, 78, 61–72.
Murphy, E. A., & Aucott, M. (1998). An assessment of the amounts of arsenical pesticides used historically in a geographical area. Science of the Total Environment, 218, 89–101.
Murphy, E. A., & Aucott, M. (1999). A methodology to assess the amounts of pesticidal mercury used historically in New Jersey. Journal of Soil Contamination, 8, 131–148.
New Jersey Department of Environmental Protection. (2005). 1930 aerial photography of New Jersey. Trenton: New Jersey Department of Environmental Protection, Office of Information Resources Management, Bureau of Geographic Information Systems. Available at: http://njgis.state.nj.us/dep/DEP_iMapNJDEP/viewer.htm.
New Jersey Department of Environmental Protection. (2011). Surface Water Quality Standards, N.J.A.C. 7:9B: Available at http://www.state.nj.us/dep/wms/bwqsa/swqs.htm.
Nolan, B. T., Baehr, A. L., & Kauffman, L. J. (2003). Spatial variability of groundwater recharge and its effect on shallow groundwater quality in southern New Jersey. Vadose Zone Journal, 2, 677–691.
Olson, M.L., & DeWild, J.F. (1999). Techniques for the collection and specific analysis of low levels of mercury in water, sediment, and biota. U.S. Geological Survey Water Resources Investigations Report 99-4018B.O’Neil, M.J., (2006). (Ed). Merck Index—14th Ed. Whitehouse Station, NJ: Merck and Co., Inc., pp. 1017–1018.
O’Neil, M.J., (2006). (Ed). Merck Index—14th Ed. Whitehouse Station, NJ: Merck and Co., Inc., pp. 1017–1018.
Pirrone, N., Cinnirella, S., Feng, X., Finkelman, R. B., Friedli, H. R., Leaner, J., et al. (2010). Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmospheric Chemistry and Physics, 10, 5951–5964.
Porvari, P., Verta, M., Munthe, J., & Haapanen, M. (2003). Forestry practices increase mercury and methylmercury output from boreal forest catchments. Environmental Science and Technology, 37, 2389–2393.
Reilly, T.J., & Baehr, A.L.(2006). Methodology to evaluate the effect of sorption in the unsaturated zone on the storage of nitrate and other ions and their transport across the water table, southern New Jersey: US Geological Survey Scientific Investigations Report 2006-5074.
Ruppel, B., Buchanan, G., Horwitz, R.J., Overbeck, P.F., Ashley, J., Velinsky, D., & Zaoudeh, L. (2010). Research Project Summary: Routine Monitoring of Toxics in Fish—Year 4—Atlantic Coastal Inland Region. New Jersey Department of Environmental Protection, Office of Science. Available at: http://www.state.nj.us/dep/dsr/2010-rps.pdf
Ryan, J. N., & Gschwend, P. M. (1990). Colloid mobilization in two Atlantic Coastal Plain aquifers: field studies. Water Resources Research, 26, 307–322.
Ryan, J. N., & Gschwend, P. M. (1994). Effect of solution chemistry on clay colloid release from an iron oxide-coated aquifer sand. Environmental Science and Technology, 28, 1717–1726.
Schuster, P. F., Shanley, J. B., Marvin-DiPasquale, M., Reddy, M. M., Aiken, G. R., Roth, D. A., et al. (2008). Mercury and organic carbon dynamics during runoff episodes from a northeastern USA Watershed. Water, Air, and Soil Pollution, 187, 89–108.
Scudder, B.C., Chasar, L.C., Wentz, D.A., Bauch, N.J., Brigham, M.E., Moran, P.W., & Krabbenhoft, D.P. (2009). Mercury in fish, bed sediment, and water from streams across the United States, 1998–2005. U.S. Geological Survey, Scientific Investigations Report 2009-5109. Available at: http://pubs.usgs.gov/sir/2009/5109.
Seigneur, C., Vijayaraghavan, K., Lohman, K., Karamchandani, P., & Scott, C. (2004). Global source attribution for mercury deposition in the United States. Environmental Science and Technology, 38, 555–569.
Shanley, J. B., Mast, M. A., Campbell, D. H., Aiken, G. R., Krabbenhoft, D. P., Hunt, R. J., et al. (2008). Comparison of total mercury and methylmercury cycling at five sites using the small watershed approach. Environmental Pollution, 154, 143–154.
Sorenson, J. A., Glass, G. E., & Schmidt, K. W. (1994). Regional patterns of wet mercury deposition. Environmental Science and Technology, 28, 2025–2032.
Szabo, Z., Rice, D. E., Plummer, L. N., Busenberg, E., Drenkard, S., & Schlosser, P. (1996). Age dating of shallow ground water with chlorofluorocarbons, tritium/helium 3, and flow path analysis, southern New Jersey Coastal Plain. Water Resources Research, 32, 1023–1038.
Szabo, Zoltan, Oden, J.H., Gibs, J., Rice, D.E., & Ding, Y. (2002). Variation in aluminum, iron, and particle concentrations in oxic ground-water samples collected by use of tangential-flow filtration with low-flow sampling. In: Proceedings of International Society for Optical Engineering. Newton (MA). 4575 Chemical and biological early warning monitoring for water, food, and ground 2001, pp. 42–61.
Szabo, Z., Barringer, J.L., Jacobsen, E., & Smith, N.P. (2010). Variability of mercury concentrations in domestic-well water, New Jersey Coastal Plain. Geological Society of America Northeastern/Southeastern Joint Meeting, 2010 Abstracts with Programs, 178. (abstract).
Watt, M.K., & Johnson, M.L. (1992). Water resources of the unconfined aquifer system of the Great Egg Harbor Basin, New Jersey 1989–90. US Geological Survey Water-Resources Investigations Report 91-4126.
Warner, K. A., Bonzongo, J. C. J., Roden, E. E., Ward, G. M., Green, A. C., Chaubey, I., et al. (2005). Effect of watershed parameters on mercury distribution in different environmental compartments in the Mobile Alabama River Basin, USA. Science of the Total Environment, 347, 187–207.
Wilde, F.D., Radtke, D.B., Gibs, J., Iwatsubo, R.T. (2004). Processing of water samples (version 2.1). U.S. Geological Survey Techniques of Water-Resources Investigations, book 9, chap. A5. Available at: http://pubs.water.usgs.gov/twri9A5/. Accessed 03 05 2008
Wilhelm, S. M. (2001). Estimate of mercury emissions to the atmosphere from petroleum. Environmental Science and Technology, 35, 4704–4710.
WSDA and Ecology. (2007). Levels of nonnutritive substances in fertilizers—Report to the Legislature 2007; Unpublished report; Washington State Departments of Agriculture and Ecology. Available at: www.agr.wa.gov. Accessed 01 08 2010
Wu, F., Cai, Y., Evans, D., & Dillon, P. (2004). Complexation between Hg(II) and dissolved organic matter in stream waters: an application of fluorescence spectroscopy. Biogeochemistry, 71, 339–351.
Yin, X., & Balogh, S. J. (2002). Mercury concentrations in stream and river water: an analytical framework applied to Minnesota and Wisconsin (USA). Water, Air, and Soil Pollution, 138, 79–100.
Yin, Y., Allen, H. E., Li, Y., Huang, C. P., & Sanders, P. F. (1996). Adsorption of mercury(II) by soil: effects of pH, chloride, and organic matter. Journal of Environmental Quality, 25, 837–844.
Acknowledgments
Funds for this study were provided by NJDEP. We gratefully acknowledge Barbara Hirst, Kimberly Cenno, Marzooq Alebus, Miroslawa Gorska, Anne Witt, Frank Lapinski, Robert Richards, and Gary Lipsius of NJDEP for help in planning the study and for supplying important information about Hg contamination in the study area. Thanks go to USGS colleagues Richard L. Walker and Robert Rosman for installing the piezometers, to Jennifer L. Bonin, Michael J. Deluca, Heather A. Heckathorn, and Daniel D. Skulski for help in sample collection, to Donald E. Rice for analysis of land use and construction of maps, and to Denis K. Sun for illustrations. We are grateful to USGS colleague Douglas A. Burns and to Aria Amirbahman (University of Maine) for their helpful reviews of drafts of this paper. (Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the US Government.)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 52.5 kb)
Rights and permissions
About this article
Cite this article
Barringer, J.L., Szabo, Z., Reilly, P.A. et al. Variable Contributions of Mercury from Groundwater to a First-Order Urban Coastal Plain Stream in New Jersey, USA. Water Air Soil Pollut 224, 1475 (2013). https://doi.org/10.1007/s11270-013-1475-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1475-7