Abstract
Artemisia vulgaris (mugwort) is a tall (1.0–2.0 m) high biomass perennial herb which accumulates considerable amounts of metals on contaminated sites. An outdoor pot experiment was conducted on a sandy, slightly alkaline soil of moderate fertility to study the uptake of cadmium and the distribution of Cd in plant tissues of A. vulgaris. Cadmium was applied as CdCl2 (a total of 1 l solution of 0, 10, 50 and 100 mg Cd l−1) to 12-l pots with a height of 25 cm. HNO3- and water-extractable concentrations of Cd were correlated with the applied Cd at 2-cm soil depth, but were not correlated at 20-cm soil depth, suggesting that Cd was either not mobile in the soil or completely taken up by mugwort roots. The Cd concentrations in different organs of A. vulgaris and litter increased with increasing soil contamination. Leaf/soil concentration ratios (BCFs) up to 65.93 ± 32.26 were observed. Translocation of Cd to the aboveground organs was very high. The leaf/root Cd concentration ratio (translocation factor) ranged from 2.07 ± 0.56 to 2.37 ± 1.31; however, there was no correlation of translocation factors to Cd enrichment, indicating similar translocation upon different soil contamination levels. In summary, A. vulgaris is tolerant to the metal concentrations accumulated, has a high metal accumulating biomass and accumulates Cd up to about 70% in the aboveground parts. Both a high phytoextraction potential and a high value for phytostabilisation would recommend mugwort for phytoremediation.
Similar content being viewed by others
1 Introduction
Phytoextraction has been defined as the green technology that removes pollutants from contaminated soil by plant absorption and translocation to harvestable plant parts (Chaney 1983; cited in Meers et al. 2010). The search for metal hyperaccumulators, i.e. plants containing >100 mg Cd per kilogram, >1,000 mg Co, Cr, Cu, Ni, Pb or Se per kilogram, or >10,000 mg Zn per kilogram dry mass in any aboveground tissue when grown in its natural habitats (Baker and Brooks 1989) has left its mark on the last decades (Baker and Whiting 2002; Ernst 2000; McGrath et al. 2002; Salt et al. 1995; Schwartz et al. 2003; Zhao et al. 2003). However, application of hyperaccumulators is limited by the generally low biomass and slow growth rate of hyperaccumulating species like Thlaspi caerulescens or Arabidopsis halleri (Meers et al. 2010; Migeon et al. 2009). Therefore, high biomass plants which concentrate considerable amounts of toxic substances an order of magnitude lower (e.g. 10–100 mg Cd per kilogram dry mass) in aboveground plant parts could be of more practical relevance.
Another plant-based remediation approach is phytostabilisation to reduce the mobility of metals in soil and in this way decrease the risk for leaching to the groundwater and for spreading of contaminants via surficial runoff and wind erosion (Vangronsveld et al. 2009).
The experience with pollutants in the context of phytoindication and monitoring (Little and Martin 1972; Murphy et al. 2000; Simon et al. 1996; Thomas et al. 1984) provides a widely unused resource for phytoremediation. Some ruderal plants growing in the vicinity of smelters or other polluted industrial areas show moderate to high levels of tolerance and accumulate toxic substances to variable degrees. For example, mugwort (Artemisia vulgaris L.) leaves intercept and accumulate high levels of heavy metals and other pollutants (Rebele 1989). We found up to 12.4 mg Cd, 2,340 mg Cu, 1,031 mg Pb and 2,301 mg Zn per kilogram dry mass in mugwort leaves in the vicinity of a copper smelter (Rebele et al. 1993).
Mugwort is a tall rhizomatous perennial hemicryptophyte which grows most frequently on disturbed urban sites, along roadsides and wastelands. The rhizomes (underground stems) range from a few millimetres to >1 cm in diameter, typically branching at the nodes, and reaching depth of 7–18 cm in the soil. The leaves are 1–10 cm long and 3–7.5 cm wide, with the upper surface being slightly hairy and the lower surface covered with silvery white wooly hairs. The leaves of the lower portion of the stem are coarsely segmented, with each segment further dissected (Barney and DiTommaso 2003). Natural habitats of mugwort are steppes, river floodplains and alluvial shrublands (Wagenitz 1987). A. vulgaris is native to Eurasia and has been introduced to North and South America and Australia (Barney and DiTommaso 2003). Globally, A. vulgaris is tolerant of a wide range of climatic conditions and is reported to occur from the high mountainous regions (3,700 m) of the Northern Himalayas to the warm temperate regions of South America (Barney and DiTommaso 2003). A. vulgaris is widely known for its medical properties and as a spice. In North America, mugwort is nowadays mostly considered as a troublesome weed (Barney and DiTommaso 2003; Weston et al. 2005), but in the past, it was also considered for the reclamation of disturbed land (Schuman and Howard 1978). Preferably in Asia, it is still in medicinal use (Barney and DiTommaso 2003); in India, it is even seen as a threatened and valuable medicinal plant and an object of biotechnological mass propagation (Govindaraj et al. 2008). Mugwort grows over a wide range of bare soils, but preferably, it grows on relatively fertile soils (Grime et al. 1988). On nutrient-rich urban soils, A. vulgaris stands are 1.5–2.0 m high and produce up to 1.6 kg above-ground dry mass per square metre (Rebele and Werner 1984).
Due to its tolerance to metal pollution and high biomass production, the question was raised whether mugwort is an appropriate plant for phytoremediation of sites contaminated by heavy metals, such as industrial wastelands or areas treated with contaminated sewage sludge.
Because field situations in industrial areas are very complex, with both aerial deposition of pollutants on aboveground plant parts and uptake of metals from contaminated soil, we conducted an outdoor pot experiment at our experimental garden with a low background level of atmospheric metal deposition.
We examined the uptake and distribution of Cd in aboveground and belowground organs of A.vulgaris, the biomass production and the relationship between the concentrations of Cd in soil and plant parts. We also calculated the total extraction of Cd from soil, the bioconcentration factor and the phytoextraction potential of A. vulgaris. We further discuss the practical use of A. vulgaris for phytoextraction of metals and phytostabilisation.
We used Cd in our experiment because of its particular concern with respect to environmental quality and health. Cd has no essential biological function and is highly toxic to animals and plants. Sources of soil contamination by Cd are the mining and smelting of zinc and cadmium ores, atmospheric pollution from metallurgical industries, the disposal of wastes containing Cd, sewage sludge application to land and the burning of fossil fuels. Agricultural soils can be contaminated via phosphatic fertilisers because relatively high contents of Cd are found in most phosphorites used for the manufacture of fertilisers. Cd tends to be more mobile in soils and therefore more available to plants than many other heavy metals (Alloway 1995). At least 70% of the Cd taken up by humans arises from plant food, although tobacco smoking and occupational exposures to CdO fumes are also important sources of the metal (Álvarez-Ayuso 2008). The background level of Cd is 0.3 mg kg−1 or less in agricultural soils (McGrath et al. 2002). Elevated Cd concentrations >1 mg kg−1 result from contamination at close proximity to natural metalliferous outcrops or as a result of mining, smelting or other industrial activities (Baker and Proctor 1990). The critical total soil Cd concentration for crop plants is 8 mg kg−1 (Alloway 1995).
2 Materials and Methods
2.1 Experimental Setup
Twenty-five 12-l pots with a diameter of 25 cm and a height of 25 cm were filled with silty sand from a ruderal site in Berlin. The substrate (Table 1) had a low humus and nitrogen content, but was Ca-rich and slightly alkaline (pH 7.5; measured in 0.01 M CaCl2). Soil bulk density was approximately 1.2 kg dm−3. Total Cd concentration at the beginning of the experiment was 0.38 mg kg−1.
Seedlings of A. vulgaris with a height of 5–8 cm were planted in spring (five plants per pot). Six weeks later, when plants covered the pot surface and had a height of 30–40 cm, CdCl2 solutions containing 0, 10, 50 and 100 mg Cd per litre were added in five portions of 200-ml in intervals of 5 days (a total of 1 l solution per pot). Five replicates were run for each treatment.
The pots were kept outdoors without additional watering at our ecological field station “Kehler Weg” in Berlin-Dahlem (52°27′ N, 13°17′ E) for 6 months (May to October). Total rainfall was about 280 mm with a drought period in late summer (August/September). The mean maximum temperature was 27.7°C and the mean minimum temperature was 6.7°C. The relative humidity during the summer months ranged between 71% and 79%. A second control with 0 Cd added was grown in a greenhouse at the field station. Greenhouse plants were watered when required. The pots in the greenhouse and in the field were allowed to drain.
2.2 Sampling and Analyses
Soil samples were taken from each pot at depths of 2 and 20 cm when the plants were harvested at the end of the experiment. The soil samples were air-dried and passed through a 2-mm sieve, and a part of each sample was ground with an agate ball mill. Total Cd concentration was measured using bomb digestion with concentrated nitric acid in Teflon cups (Kotz et al. 1972). Also water-extractable concentrations were examined because they represent the most mobile fraction, which is a crude approximation of the true soil solution (Ernst et al. 1974; Hunter et al. 1987). The water-soluble heavy metals were extracted by shaking a mixture of 4 g soil and 40 ml distilled water for 48 h at room temperature. The suspensions were then centrifuged, the supernatants filtered and the clear extracts used for the determination of Cd.
At harvest, plants had a height of 1.0–1.2 m. The plants of each pot were bulked and separated in roots, rhizomes, leaves, stems, flowers (Fig. 1) and litter (mainly dead leaves). Roots and rhizomes were washed with deionised water. After drying at 80°C for 48 h, biomass of the different tissues was determined (grams dry mass/pot). Flowers of the five pots each were bulked because dry mass of flowers was too low to be analysed at the pot level.
Cd concentrations in soil and plant material was analysed by atomic absorption spectrometry (AAS) with graphite furnace (Perkin Elmer 1100 B; Perkin Elmer Corporation, Norwalk, CT, USA).
2.3 Calculations and Statistical Analyses
Total contents of Cd in different organs were calculated by multiplying Cd concentrations of each plant part by the dry mass of each organ. Total uptake of Cd by plants of each pot was calculated by summarising the contents of aboveground and belowground organs. Also, total content of litter was calculated by multiplying Cd concentrations by litter biomass.
Plant/soil concentration ratios (bioconcentration factors or BCFs) were calculated for each plant part and litter related to the two soil depths (2 and 20 cm, respectively).
The translocation factor was calculated as the ratio of Cd concentration in leaves to that in roots (leaf/root concentration ratio). The ratio of the total Cd contents of aboveground/belowground biomass was also calculated.
The phytoextraction potential was calculated as follows: per cent of soil Cd removed by \( A.\,vulgaris = {1}00 \times ({\hbox{total content of Cd in aboveground organs}} + {\hbox{litter}})/{\hbox{total Cd soil content}} \).
Soil and plant Cd concentrations, biomass, total Cd content per pot and the plant/soil concentration ratios (BCFs) were analysed by univariate ANOVA and post hoc Duncan’s multiple range test. Differences between the two controls were analysed by t test.
The relationship between HNO3- and water-extractable concentrations of Cd in soil and the relationships between soil Cd concentrations and plant Cd concentrations were tested by regression analysis.
Statistics were performed using SPSS (Statistical Package for the Social Sciences) version 14 (SPSS Inc.) software packages.
3 Results
3.1 Soil Cd Concentrations
HNO3- and water-extractable concentrations of Cd at 2-cm soil depth were significantly correlated with the applied Cd (Table 2). There was also a clear linear relationship between water-extractable and total Cd in the soil for the 2-cm depth (r = 0.893, p < 0.001). Total Cd concentrations at the 2-cm depth ranged from 0.44 ± 0.02 (control) to 17.13 ± 11.21 mg kg−1 (+100 mg Cd added).
HNO3-extractable concentrations of Cd at the 20-cm soil depth were not significantly correlated with the applied Cd (Table 2). Total Cd concentrations ranged from 0.34 ± 0.01 (control) to 0.42 ± 0.10 mg kg−1 (+100 mg Cd; Table 2). No Cd was detectable in the water extract at all levels of Cd enrichment.
HNO3-extractable Cd concentrations of soil samples from the controls (outdoor vs. greenhouse) were not significantly different (t test, p < 0.05).
3.2 Cd Concentrations in Plant Organs and Litter
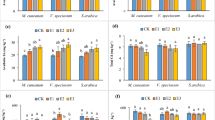
Cd concentrations in different organs (roots, rhizomes, stems, leaves, flowers) of A. vulgaris and litter increased with increasing soil contamination (Table 3). Rhizomes, stems and leaves accumulated more Cd than roots. Cd concentrations in rhizomes ranged from 0.93 ± 0.19 (control) to 28.04 ± 7.43 mg kg−1 (+100 mg Cd), in stems from 1.97 ± 0.17 (control) to 26.24 ± 4.90 mg kg−1 (+100 mg Cd), in leaves from 1.27 ± 0.30 (control) to 25.36 ± 6.14 mg kg−1 (+100 mg Cd) and in roots from 0.58 ± 12.64 (control) to 12.64 ± 2.73 mg kg−1 (+100 mg Cd; Table 3). Cd concentrations of flowers were lower than leaf concentrations, but higher than root concentrations. Cd concentrations of the litter were about twofold of the concentrations of leaves and stems at the highest contamination level (53.00 ± 11.64 mg kg−1).
All Cd concentrations of plant parts and litter were significantly correlated with the HNO3- and water-extractable concentrations of Cd in the soil from the depth of 2 cm, but were not correlated with soil concentrations at the 20-cm depth (Table 4).
Cd concentrations in different organs and litter were higher in control plants grown in the field compared to greenhouse plants (Table 5). However, the differences between the two control sets were low in relation to the differences between plants in pots with additional Cd and were not significant for leaves.
3.3 Influence of Cd on Biomass Production
Biomass production was not negatively affected by soil contamination with Cd. Total plant biomass was even highest at the highest contamination level, mainly due to a higher stem biomass (Table 6).
Generally, root biomass was greater than stem biomass, followed by rhizome and leaf biomass. Litter biomass was about one third of the living aboveground biomass.
3.4 Bioconcentration Factor
Plant/soil concentration ratios where calculated separately for the two soil depths. The leaves/soil2 cm concentration ratio ranged from 2.21 ± 1.78 (+100 mg Cd) to 4.45 ± 3.16 (+10 mg Cd), but there was no significant difference among treatments (Table 7). BCFs for other organs were also not significantly different, except for two cases of stem/soil2 cm concentration ratios.
In contrast to that, leaves/soil20 cm concentration ratios were significantly different between higher and lower contamination levels (Table 8). The leaves/soil20 cm concentration ratio ranged from 3.70 ± 0.87 (control) to 65.93 ± 32.26 (+100 mg Cd). Similar results were obtained for stem/soil, root/soil, rhizome/soil and litter/soil concentration ratios (related to a soil depth of 20 cm). The highest concentration ratio was found for litter/soil20 cm (up to 134.59 ± 49.55 in pots enriched with 100 mg Cd).
3.5 Cd Uptake and Translocation
Contents of Cd increased significantly in all plant organs from lower to higher contamination levels (Table 9). The total Cd uptake (total plant + litter) ranged from 0.019 ± 0.006 mg/pot (control) to 0.438 ± 0.086 mg/pot (+100 mg Cd; Table 10).
The translocation to the aboveground organs was very high. The leaf/root Cd concentration ratio (translocation factor) ranged from 2.07 ± 0.56 (+100 mg Cd) to 2.37 ± 1.31 (Table 11). However, there was no correlation of translocation factors to Cd enrichment, indicating similar translocation upon different soil contamination levels. The ratios of the total contents of aboveground/belowground biomass were also not correlated to soil Cd contamination (Table 11).
3.6 Phytoextraction Potential
The phytoextraction potential of A. vulgaris ranged from 0.28% to 0.33%, but there were no significant differences between contamination levels (Table 12).
4 Discussion
4.1 Soil Contamination
The Cd concentrations in the unenriched substrate of the pot study (0.34 ± 0.01 in 20 cm and 0.44 ± 0.02 mg kg−1 in 2 cm, respectively) were within the normal range of forest soils in Northern Germany where up to 0.55 mg Cd per kilogram were found in A horizons (Blume 1981). The maximum mean soil2 cm Cd concentration of pots enriched with 100 mg Cd (17.13 ± 11.21 mg kg−1) was at the upper range of contaminated urban soils in Berlin (Senatsverwaltung für Stadtentwicklung und Umweltschutz 1993) and comparable to contaminated field sites in other studies (e.g. Jensen et al. 2009; Migeon et al. 2009). In most pot experiments with Cd enrichment, Cd was mixed with the substrate, whereas in our experiment, Cd was added by solution simulating more realistic field conditions. On non-agricultural polluted soils, Cd concentrations normally decrease with depth (e.g. Dahmani-Muller et al. 2000; Rebele et al. 1993).
The evidence that Cd enrichment did not result in higher total soil Cd levels at 20-cm depth suggests that Cd was either not mobile in the soil during one vegetation period or was completely taken up by A. vulgaris. The high Cd concentrations in plant materials suggest that Cd was taken up by mugwort roots until it moves to deeper zones within the pot. Thus A. vulgaris is very effective in preventing metal leaching into soil. A former pot experiment with the rhizomatous grass Calamagrostis epigejos using the same substrate, however lasting 28 months, revealed Cd mobility in soil despite the relatively high pH of 7.5 (Lehmann and Rebele 2004).
4.2 Cd Accumulation and Transfer Characteristics
A. vulgaris accumulates Cd preferably in rhizomes, stems and leaves. The even higher Cd concentrations in litter (up to 53 ± 11.64 mg kg−1) are a result of higher Cd levels in older leaves and stems. The slightly higher Cd concentrations in plant material and litter from outdoor control plants compared to greenhouse plants may be due to a low additional aerial Cd deposition. However, outdoor plants may also have a higher transpiration rate and thus also different uptake and translocation characteristics.
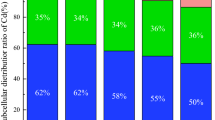
Cd concentrations of A. vulgaris in our pot experiment exceed leaf Cd concentrations found in the field around smelters or industrial areas where soil and plant contamination is mainly a result of aerial deposition (Rebele 1986; Rebele et al. 1993). The linear relationship of increasing plant Cd concentration with increasing soil contamination shows that A. vulgaris effectively extracts Cd from soil at any contamination level tested despite the slightly alkaline soil conditions. A. vulgaris also translocates Cd to harvestable plant parts. Both living and dead plant material aboveground can be harvested and subjected to further treatment. In our study 62–77% of the total plant Cd was accumulated in the aboveground organs and litter of mugwort. A. vulgaris can be characterised as an accumulator where metals are concentrated in aboveground plant parts from low or high soil levels (Baker 1981). A minor part of Cd remains in roots and rhizomes belowground and in that way also contributes to the phytostabilisation of contaminated soil.
The plant/soil2 cm Cd bioconcentration factors up to 6.37 ± 4.01 for stems are comparable or higher than BCFs in field trials with woody species or agricultural crops (e.g. Migeon et al. 2009; Vangronsveld et al. 2009). The high plant/soil20 cm Cd concentration ratios up to 68.02 ± 29.71 for stems, 65.93 ± 32.93 for leaves and 134.59 ± 49.55 for litter are within categories typical for hyperaccumulators (e.g. Zhao et al. 2003). These contrasting results for BCFs reveal a problem of field trials. Plants take up metals from different rooting zones with different soil metal concentrations (Keller et al. 2003), and thus, the point of reference may vary due to the uneven vertical distribution of metals in soil.
4.3 Biomass Production and Phytoextraction Potential
The result that biomass production was not negatively affected by Cd enrichment shows that A. vulgaris is relatively tolerant to Cd. Total biomass was even significantly higher at the highest contamination level. This result seems paradox; however, a stimulating effect of Cd on biomass production was already found in a study on the bioavailability of Cd for Triticum aestivum in nutrient solutions. Wheat plants which grew in nutrient solution five times the normal strength of Hoaglands’ solution with Cd had a higher turgor potential and a higher dry weight than those which grew in five times Hoaglands’ solution without Cd (Pearson and Kirkham 1981; cited in Kirkham 2006). The authors suggested that Cd apparently increased the permeability of membranes to ions and water because osmotic potentials were usually lower, and turgor potentials higher, with Cd than without.
In our pot experiment, we used a relatively infertile soil, so the mean total dry mass was approximately 0.362 kg m−1 during one growing season. This is low compared to A. vulgaris field stands on nutrient-rich soils where productivity is much higher. At a productive urban wasteland site, Rebele and Werner (1984) found a standing biomass of A. vulgaris with 1.601 kg m−1 aboveground and 0.440 kg m−1 belowground. Similar results were reported from urban sites in Brussels (Duvigneaud 1975).
The mean phytoextraction potential of approximately 0.3% within one growing season in our experiment is comparable to other studies of phytoextraction, e.g. with willows on calcareous urban soils (Jensen et al. 2009). However, on nutrient-rich sites (e.g. former sewage fields or arable land treated with contaminated sewage sludge) where aboveground dry mass would be about four to five times higher, the phytoextraction potential of A. vulgaris could probably amount 1.2–1.5%. Assuming equal uptake rates and continuous harvesting, it would take approximately 33–42 growing seasons to remove half of the total Cd from soil. This is longer than it was expected for hyperaccumulators, which should preferably extract metals within 10 years to tolerable contamination levels, but shorter than calculations of many other scenarios using non-hyperaccumulators (Vangronsveld et al. 2009).
4.4 Phytostabilisation
Although the phytoextraction potential of A. vulgaris is within the range, where an application for phytoextraction of Cd could be suggested, another important advantage is its value for phytostabilisation of highly contaminated sites. The possibility that A. vulgaris takes up Cd until it moves to deeper soil horizons would prevent leaching and would reduce the amount of bioavailable Cd in soil solution. A vegetation cover dominated by the perennial mugwort can also prevent spreading of polluted soil and dust aboveground. Risk-managed phytostabilisation and monitored natural attenuation becomes more and more an alternative for large-scale phytoremediation in urban and industrial areas (Dickinson et al. 2009).
5 Conclusions
In the last years, phytoextraction with willows and poplars (Klang-Westin and Eriksson 2003; Landberg and Greger 1996; Pulford et al. 2002; Pulford and Watson 2003) or non-food agricultural crops, e.g. maize for energy production (Meers et al. 2010), was recommended. Phytoextraction with A. vulgaris may be an alternative on sites where cultivation of woody species may be difficult or on multimetallic polluted sites. A perennial plant may also have an advantage over an annual crop like maize where soil remains uncovered for half a year and is thus more susceptible to leaching and erosion. In addition, A. vulgaris does not suffer from toxicity problems as many crops do.
In summary, A. vulgaris is tolerant to the metal concentrations accumulated, has a high value for phytostabilisation, has a high metal accumulating biomass and accumulates Cd up to about 70% in the aboveground parts.
Mugwort is easy to cultivate and harvest and can probably provide an added economic advantage. Medicinal use is less likely on highly contaminated sites, but the biomass of A. vulgaris may be used as a non-food renewable energy crop. The low lignin but high protein content of mugwort (Schuman and Howard 1978) would be advantageous for anaerobic digestion and conversion into biogas.
References
Alloway, B. J. (1995). Heavy metals in soils (2nd ed.). Glasgow: Blackie Academic and Professional.
Álvarez-Ayuso, E. (2008). Cadmium in soil–plant systems: an overview. International Journal of Environment and Pollution, 33, 275–291.
Baker, A. J. M. (1981). Accumulators and excluders—Strategies in the response of plants to heavy metals. Journal of Plant Nutrition, 3, 643–654.
Baker, A. J. M., & Brooks, R. R. (1989). Terrestrial higher plants which can hyperaccumulate metallic elements—A review of their distribution, ecology and phytochemistry. Biorecovery, 1, 81–126.
Baker, A. J. M., & Proctor, J. (1990). The influence of cadmium, copper, lead, and zinc on the distribution and evolution of metallophytes in the British Isles. Plant Systematics and Evolution, 173, 91–108.
Baker, A. J. M., & Whiting, S. N. (2002). In search of the Holy Grail—A further step in understanding metal hyperaccumulation? The New Phytologist, 155, 1–4.
Barney, J. N., & DiTommaso, A. (2003). The biology of Canadian weeds. 118. Artemisia vulgaris L. Canadian Journal of Plant Science, 83, 205–215.
Blume, H. P. (1981). Schwermetallverteilung und-bilanzen typischer Waldböden aus nordischem Geschiebemergel. Zeitschrift für Pflanzenernährung und Bodenkunde, 144, 156–163.
Chaney, R. L. (1983). Plant uptake of inorganic waste constituents. In J. F. Parr, P. B. Marsh, & J. M. Kla (Eds.), Land treatment of hazardous waste (pp. 50–76). Parkridge: Noyes Data Corporation.
Dahmani-Muller, H., van Oort, F., Gélie, B., & Balabane, M. (2000). Strategies of heavy metal uptake by three plant species growing near a metal smelter. Environmental Pollution, 109, 231–238.
Dickinson, N. M., Baker, A. J. M., Doronila, A., Laidlaw, S., & Reeves, R. D. (2009). Phytoremediation of inorganics: Realism and synergies. International Journal of Phytoremediation, 11, 97–114.
Duvigneaud, P. (1975). Structure, biomasses, minéralomasses, productivité et captation du plomb dans quelques associations rudérales (Artemisietalia vulgaris). Bulletin de la Société Royale de Botaniquie Belgique, 108, 93–128.
Ernst, W. H. O. (2000). Evolution of metal hyperaccumulation and phytoremediation hype. The New Phytologist, 146, 357–358.
Ernst, W. H. O., Mathys, W., Salaske, J., & Janiesch, P. (1974). Aspekte von Schwermetallbelastungen in Westfalen. Abhandlungen des Landesmuseums für Naturkunde Münster, 36(2), 1–33.
Govindaraj, S., Kumari, B. D. R., Cioni, P. L., & Flamini, G. (2008). Mass propagation and essential oil analysis of Artemisia vulgaris. Journal of Bioscience and Bioengineering, 105, 176–183.
Grime, J. P., Hodgson, J. G., & Hunt, R. (1988). Comparative plant ecology. London: Unwin Hyman.
Hunter, B. A., Johnson, M. S., & Thompson, D. J. (1987). Ecotoxicology of copper and cadmium in a contaminated grassland ecosystem. Journal of Applied Ecology, 24, 573–586.
Jensen, J. K., Holm, P. E., Nejrup, J., Larsen, M. B., & Borggaard, O. K. (2009). The potential of willow for remediation of heavy metal polluted calcareous urban soils. Environmental Pollution, 157, 931–937.
Keller, C., Hammer, D., Kayser, A., Richner, W., Brodbeck, M., & Sennhauser, M. (2003). Root development and heavy metal phytoextraction efficiency: Comparison of different plant species in the field. Plant and Soil, 249, 67–81.
Kirkham, M. B. (2006). Cadmium in plants on polluted soils: Effects of soil factors, hyperaccumulation, and amendments. Geoderma, 137, 19–32.
Klang-Westin, E., & Eriksson, J. (2003). Potential of Salix as phytoextractor for Cd on moderately contaminated soils. Plant and Soil, 249, 127–137.
Kotz, L., Kaiser, G., Tschöpel, P., & Tölg, G. (1972). Aufschluß biologischer Matrices für die Bestimmung sehr niedriger Spurenelementgehalte bei begrenzter Einwaage mit Salpetersäure in einem Teflongefäß. Zeitschrift für Analytische Chemie, 260, 207–209.
Landberg, T., & Greger, M. (1996). Differences in uptake and tolerance to heavy metals in Salix from unpolluted and polluted areas. Applied Geochemistry, 11, 175–180.
Lehmann, C., & Rebele, F. (2004). Assessing the potential for cadmium phytoremediation with Calamagrostis epigejos: A pot experiment. International Journal of Phytoremediation, 6, 169–183.
Little, P., & Martin, M. H. (1972). A survey of zinc, lead and cadmium in soil and natural vegetation around a smelting complex. Environmental Pollution, 3, 241–254.
McGrath, S. P., Zhao, F. J., & Lombi, E. (2002). Phytoremediation of metals, metalloids, and radionuclides. Advances in Agronomy, 75, 1–56.
Meers, E., Van Slycken, S., Adriaensen, K., Ruttens, A., Vangronsveld, J., Du Laing, G., et al. (2010). The use of bio-energy crops (Zea mays) for ‘phytoattenuation’ of heavy metals on moderately contaminated soils: A field experiment. Chemosphere, 78, 35–41.
Migeon, A., Richaud, P., Guinet, F., Chalot, M., & Blaudez, D. (2009). Metal accumulation by woody species on contaminated sites in the north of France. Water, Air, and Soil Pollution, 204, 89–101.
Murphy, A. P., Coudert, M., & Barker, J. (2000). Plants as biomarkers for monitoring heavy metal contaminants on landfill sites using sequential extraction and inductively coupled plasma atomic emission spectrophotometry (ICP-AES). Journal of Environmental Monitoring, 2, 621–627.
Pearson, C. H., & Kirkham, M. B. (1981). Water relations of wheat cultivars grown with cadmium. Journal of Plant Nutrition, 3, 309–318.
Pulford, I. D., & Watson, C. (2003). Phytoremediation of heavy metal-contaminated land by trees—A review. Environment International, 29, 529–540.
Pulford, I. D., Riddell-Black, D., & Stewart, C. (2002). Heavy metal uptake by willow clones from sewage sludge-treated soil: The potential for phytoremediation. International Journal of Phytoremediation, 4, 59–72.
Rebele, F. (1986). Die Ruderalvegetation der Industriegebiete von Berlin (West) und deren Immissionsbelastung. Landschaftsentwicklung und Umweltforschung, 43, 1–224.
Rebele, F. (1989). Ruderal plants as bioindicators in the industrial areas of Westberlin. In J. Boháč and V. Ružička (Eds.) Proceedings of the Vth International Conference Bioindicatores deteriorisationis regionis, České Budějovice 1988, pp. 44–54.
Rebele, F., & Werner, P. (1984). Untersuchungen zur ökologischen Bedeutung industrieller Brach-und Restflächen in Berlin (West). Berlin: Freie Universität Berlin.
Rebele, F., Surma, A., Kuznik, C., Bornkamm, R., & Brej, T. (1993). Heavy metal contamination of spontaneous vegetation and soil around the copper smelter “Legnica”. Acta Societatis Botanicorum Poloniae, 62, 53–57.
Salt, D. E., Blaylock, M., Kumar, N. P. B. A., Dushenkov, V., Ensley, B. D., Chet, I., et al. (1995). Phytoremediation: A novel strategy for the removal of toxic metals from the environment using plants. Biotechnology, 13, 468–474.
Schuman, G. E., & Howard, G. S. (1978). Artemisia vulgaris L.: An ornamental plant for disturbed land reclamation. Journal of Range Management, 31, 392–393.
Schwartz, C., Echevarria, G., & Morel, J. L. (2003). Phytoextraction of cadmium with Thlaspi caerulescens. Plant and Soil, 249, 27–35.
Senatsverwaltung für Stadtentwicklung und Umweltschutz (Ed.) (1993). Umweltatlas Berlin, Bd. 1. Berlin: Kulturbuch.
Simon, L., Martin, H. W., & Adriano, D. C. (1996). Chicory (Cichorium intybus L.) and dandelion (Taraxacum officinale Web.) as phytoindicators of cadmium contamination. Water, Air, and Soil Pollution, 91, 351–362.
Thomas, W., Rühling, Å., & Simon, H. (1984). Accumulation of airborne pollutants (PAH, chlorinated hydrocarbons, heavy metals) in various plant species and humus. Environmental Pollution (Series A), 36, 295–310.
Vangronsveld, J., Herzig, R., Weyens, N., Boulet, J., Adriansen, K., Ruttens, A., et al. (2009). Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environmental Science and Pollution Research, 16, 765–794.
Wagenitz, G. (1987). Gustav Hegi, Illustierte Flora von Mitteleuropa, Band 4, Teil 4 (2nd ed.). Berlin: Verlag Paul Parey.
Weston, L. A., Barney, J. N., & DiTommaso, A. (2005). A review of the biology and ecology of three invasive perennials in New York State: Japanese knotweed (Polygonum cuspidatum), mugwort (Artemisia vulgaris) and pale swallow-wort (Vincetoxicum rossicum). Plant and Soil, 277, 53–69.
Zhao, F. J., Lombi, E., & McGrath, S. P. (2003). Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator Thlaspi caerulescens. Plant and Soil, 249, 37–43.
Acknowledgements
We thank an anonymous reviewer for helpful comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rebele, F., Lehmann, C. Phytoextraction of Cadmium and Phytostabilisation with Mugwort (Artemisia vulgaris). Water Air Soil Pollut 216, 93–103 (2011). https://doi.org/10.1007/s11270-010-0517-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-010-0517-7