Abstract
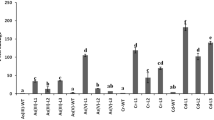
As most cultivars of flax and linseed (Linum usitatissimum L.) are capable of accumulating cadmium (Cd), they are suitable candidates for phytoextraction of the metal from contaminated soils. In an attempt to enhance the phytoextraction capacity of L. usitatissimum through overproduction of an efficient heterologous Cd-binding peptide, we engineered linseed breeding line AGT 917 to constitutively express genetic fusion of α-domain of mammalian metallothionein 1a (αMT1a) and β-glucuronidase gus gene under the control of CaMV 35S promoter. An improved transformation protocol was developed, which involved co-cultivation of AGT 917 hypocotyl segments with partially removed epidermis with Agrobacterium suspension for 10 min in the presence of 200 mg l−1 cellulase. The enzyme treatment increased the transformation efficiency (TE) 1.6-fold as compared to agroinfection without cellulase. Less pronounced impact on TE exerted 100 mg l−1 acetosyringone, increasing TE 1.3-fold. When tested in soils amended with Cd at 20 and 360 mg kg−1, the mature αMT1a::gus plants accumulated more Cd than parental AGT 917: the stem Cd concentrations in the best performing αMT1/2 line were 3.3- and 1.9-fold higher, respectively. Moreover, hypocotyl explants of αMT1/2 line showed 1.7-fold higher biomass than those of AGT 917 on media containing 15 mg Cd l−1, indicating that αMT1a::gus did confer higher Cd tolerance to engineered plant. Overproduction of metal-binding peptides thus appears to be a viable strategy for the production of L. usitatissimum with improved phytoremediation capacity.





Similar content being viewed by others
References
Angelova V, Ivanova R, Delibaltova V, Ivanov K (2004) Bio-accumulation and distribution of heavy metals in fibre crops (flax, cotton and hemp). Ind Crop Prod 19:197–205
Batra S, Kumar S (2003) Agrobacterium-mediated transient GUS gene expression in buffel grass (Cenchus ciliaris L.). J Appl Genet 44:449–458
Bennet LE, Burkhead JL, Hale KL, Terry N, Pilon M, Pilon-Smits EAH (2003) Analysis of transgenic Indian mustard plants for phytoremediation of metal-contaminated mine tailings. J Environ Qual 32:432–440
Beranová M, Rakouský S, Vávrová Z, Skalický T (2008) Sonication assisted Agrobacterium–mediated transformation enhances the transformation efficiency in flax (Linum usitatissimum L.). Plant Cell Tiss Organ Cult 94:253–259
Bertin G, Averbeck D (2006) Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 88:1549–1559
Bhargava A, Carmona FF, Bhargava M, Srivastava S (2012) Approaches for enhanced phytoextraction of heavy metals. J Environ Manag 105:103–120
Bhuiyan MSU, Min SR, Jeong WJ, Sultana S, Choi KS, Lee Y, Liu JR (2011a) Overexpression of AtATM3 in Brassica juncea confers enhanced heavy metal tolerance and accumulation. Plant Cell Tiss Organ Cult 107:69–77
Bhuiyan MSU, Min SR, Jeong WJ, Sultana S, Choi KS, Song WY, Lee Y, Lim YP, Liu JR (2011b) Overexpression of a yeast cadmium factor 1 (YCF1) enhances heavy metal tolerance and accumulation in Brassica juncea. Plant Cell Tiss Organ Cult 105:85–91
Bjelková M, Genčurová V, Griga M (2011) Accumulation of cadmium by flax and linseed cultivars in field-simulated conditions: a potential for phytoremediation of Cd-contaminated soils. Ind Crop Prod 33:761–774
Bretagne B, Chupeau MC, Chupeau Y, Fouilloux G (1994) Improved flax regeneration from hypocotyls using thidiazuron as a cytokinin source. Plant Cell Rep 14:120–124
Broadley MR, Willey NJ, Wilkins JC, Baker AJM, Mead A, White PJ (2001) Phylogenetic variation in heavy metal accumulation in angiosperms. New Phytol 152:9–27
Buendía-Gonzáles L, Estrada-Zúnñiga ME, Orozco-Villafuerte J, Croz-Sosa F, Vernon-Carter EJ (2012) Somatic embryogenesis of the heavy metal accumulator Prosopis laevigata. Plant Cell Tiss Organ Cult 108:287–296
Chaney RL, Angle JS, McIntosh MS, Reeves RD, Li YM, Brewer EP, Chen K-Y, Rosenberg RJ, Perner H, Synkowski EC, Broadhurst CL, Wang S, Baker AJM (2005) Using hyperaccumulator plants to phytoextract soil Ni and Cd. Z Naturforsch 60:190–198
Clemens S (2006) Toxic metal accumulation, responses to exposures and mechanisms of tolerance in plants. Biochimie 88:1707–1719
Costa M, Miguel C, Oliveira M (2006) An improved selection strategy and the use of acetosyringone in shoot induction medium increase almond transformation efficiency by 100-fold. Plant Cell Tiss Organ Cult 85:205–209
Coyle P, Philcox JC, Carey LC, Rofe AM (2002) Metallothionein: the multipurpose protein. Cell Mol Life Sci 59:627–647
de Borne FD, Elmayan T, de Roton C, de Hys L, Tepfer M (1998) Cadmium partitioning in transgenic tobacco plants expressing a mammalian metallothionein gene. Mol Breeding 4:83–90
De Clerc J, Zambre M, Van Montagu M, Dillen W, Angenon G (2002) An optimized Agrobacterium-mediated transformation procedure for Phaseolus acutifolius A. Gray. Plant Cell Rep 21:333–340
Dong JZ, McHughen A (1993) An improved procedure for production of transgenic flax plants using Agrobacterium tumefaciens. Plant Sci 88:61–71
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
François IEJA, De Bolle MFC, Dwyer G, Goderis IJWM, Woutors PFJ, Verhaert PD, Proost P, Schaaper WMM, Cammue BPA, Broekaert WF (2002) Transgenic expression in Arabidopsis of a polyprotein construct leading to production of two different antimicrobial proteins. Plant Physiol 128:1346–1358
Freisinger E (2008) Plant MTs-long neglected members of the metallothionein superfamily. Dalton Trans 47:6663–6675
Gasic K, Korban SS (2007) Transgenic Indian mustard (Brassica juncea) plants expressing an Arabidopsis phytochelatin synthase (AtPCS1) exhibit enhanced As and Cd tolerance. Plant Mol Biol 64:361–369
Gelvin SB (2003) Agrobacterium-mediated plant transformation: the biology behind the “Gene-Jockyeing” tool. Microbiol Mol Biol Rev 67:16–37
Hansen G, Wright M (1999) Recent advances in the transformation of plants. Trends Plant Sci 4:226–231
Hood EE, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res 2:208–218
Jiao Y, Grant CA, Bailey LD (2004) Effects of phosphorus and zinc fertilizer on cadmium uptake and distribution in flax and durum wheat. J Sci Food Agric 84:777–785
Jordan MC, McHughen A (1988) Glyphosate tolerant flax plants from Agrobacterium mediated gene transfer. Plant Cell Rep 7:281–284
Kabata-Pendias A (2011) Trace elements in soils and plants. CRC Press, Boca Raton
Kotrba P, Pospíšil P, de Lorenzo V, Ruml T (1999) Enhanced metallosorption of Escherichia coli cells due to surface display of β- and α-domains of mammalian metallothionein as a fusion to LamB protein. J Recept Signal Transduct Res 19:703–715
Kotrba P, Najmanova J, Macek T, Ruml T, Mackova M (2009) Genetically modified plants in phytoremediation of heavy metal and metalloid soil and sediment pollution. Biotech Adv 27:799–810
Krämer U (2010) Metal hyperaccumulation in plants. Annu Rev Plant Biol 61:517–534
Lux A, Martinka M, Vaculík M, White PJ (2011) Root responses to cadmium in the rhizosphere: a review. J Exp Bot 62:21–37
Macek T, Macková M, Pavlíková D, Száková J, Truksa M, Singh Cundy A, Kotrba P, Yancey N, Scouten WH (2002) Accumulation of cadmium by transgenic tabacco. Acta Biotechnol 22:101–106
Meyer C-L, Verbruggen N (2012) The use of the model species Arabidopsis halleri towards phytoextraction of cadmium polluted soils. N Biotechnol. doi:10.1016/j.nbt.2012.07.009
Moulis JM (2010) Cellular mechanisms of cadmium toxicity related to the homeostasis of essential metals. Biometals 23:877–896
Najmanova J, Neumannova E, Leonhardt T, Zitka O, Kizek R, Macek T, Macková M, Kotrba P (2012) Cadmium-induced production of phytochelatins and speciation of intracellular cadmium in organs of Linum usitatissimum seedlings. Ind Crop Prod 36:536–542
Okamoto M, Mitsuhara I, Ohshima M, Natori S, Ohashi Y (1998) Enhanced expression of an antimicrobial peptide sarcotoxin IA by GUS fusion in transgenic tobacco plants. Plant Cell Physiol 39:57–63
Pilon-Smits E (2005) Phytoremediation. Annu Rev Plant Biol 56:15–39
Rakouský S, Tejklová E, Wiesner I, Wiesnerová D, Kocábek T, Ondřej M (1999) Hygromycin B—an alternative in flax transformation selection. Biol Plant 42:361–369
Richau KH, Kozhevnikova AD, Seregin IV, Vooijs R, Koevoets PLM, Smith JAC, Ivanov VB, Schat H (2009) Chelation by histidine inhibits the vacuolar sequestration of nickel in roots of the hyperaccumulator Thlaspi caerulescens. New Phytol 183:106–116
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. ISBN 0-87969-577-3
Sarwar N, Saifullah, Malhi SS, Zia MH, Naeem A, Bibi S, Farid G (2010) Role of mineral nutrition in minimizing cadmium accumulation by plants. Sci Food Agric 90:925–937
Shi G, Cai Q (2009) Cadmium tolerance and accumulation in eight potential energy crops. Biotechnol Adv 27:555–561
Singh KK, Mridula D, Rehal J, Barnwal P (2011) Flaxseed: a potential source of food, feed and fiber. Crit Rev Food Sci Nutr 51:210–222
Smýkalová I, Vrbová M, Tejklová E, Větrovcová M, Griga M (2010) Large scale screening of heavy metal tolerance in flax/linseed (Linum usitatissimum L.) tested in vitro. Ind Crops Prod 32:527–533
Soudek P, Katrušáková A, Sedláček A, Petrová Š, Kočí V, Maršík P, Griga M, Vaněk T (2010) Effect of heavy metals on inhibition of root elongation in 23 cultivars of flax (Linum usitatissimum L.). Arch Environ Con Tox 59:194–203
Stillman MJ, Cai W, Zelazowski AJ (1987) Cadmium binding to metallothioneins. Domain specificity in reactions of alpha and beta fragments, apometallothionein and zinc metallothionein with Cd2+. J Biol Chem 262:4538–4548
Švábová L, Griga M (2008) The effect of cocultivation treatments on transformation efficiency in pea (Pisum sativum L.). Plant Cell Tiss Organ Cult 95:293–304
Tzfira T, Citovsky V (2006) Agrobacterium-mediated genetic transformation of plants: biology and biotechnology. Curr Opin Biotechnol 17:147–154
Wangeline AL, Burkhead JL, Hale KL, Lindblom SD, Terry N, Pilon M, Pilon-Smits EAH (2004) Overexpression of ATP Sulfurylase in Indian mustard: effects on tolerance and accumulation of twelve metals. J Environ Qual 33:54–60
Weber S, Friedt W, Landes N, Molinier J, Himber C, Rousselin P, Hahne G, Horn R (2003) Improved Agrobacterium-mediated transformation of sunflower (Heliantus annuus L.): assessment of macerating enzymes and sonication. Plant Cell Rep 21:475–482
Xing Y, Yang Q, Ji Q, Luo Y, Zhang Y, Gu K, Wang D (2007) Optimization of Agrobacterium-mediated transformation parameters for sweet potato embryogenic callus using β-glucuronidase (GUS) as a reporter. African J Biotech 6:2578–2584
Xiong Y, Chen Y, Ru B (1998) The expressed alpha domain of mouse metallothionein-I from Escherichia coli displays independent structure and function. Biochem Mol Biol Int 46:307–319
Xu J, Chai T, Zhang Y, Lang M, Han L (2009) The cation-efflux transporter BjCET2 mediates zinc and cadmium accumulation in Brassica juncea L. leaves. Plant Cell Rep 28:1235–1242
Yasuda H, Tada Y, Hayashi Y, Jomori T, Takaiwa F (2005) Expression of the small peptide GLP-1 in transgenic plants. Transgenic Res 14:677–684
Zhan X, Jones DA, Kerr A (1988) Regeneration of flax plants transformed by Agrobacterium rhizogenes. Plant Mol Biol 11:551–559
Zhao S-J, Zhang Z-C, Gao X, Tohsun G, Qui B-S (2009) Plant regeneration of the mining ecotype Sedum alfredii and cadmium hyperaccumulation in regenerated plants. Plant Cell Tiss Organ Cult 99:9–16
Zhu YL, Pilon-Smits EAH, Jouanin L, Terry N (1999a) Overexpression of glutathione synthetase in Indian mustard enhances cadmium accumulation and tolerance. Plant Physiol 119:73–79
Zhu YL, Pilon-Smits EAH, Tarun AS, Weber SU, Jouanin L, Terry N (1999b) Cadmium tolerance and accumulation in Indian mustard is enhanced by overexpressing γ-glutamylcysteine synthetase. Plant Physiol 121:1169–1177
Acknowledgments
This work was supported by grants from the Ministry of Education, Youth and Sports of the Czech Republic (no. 2678424601 and 1M06030). The authors thank Dr. E. Tejklová for providing seeds of linseed breeding lines. We are also grateful to J. Matulíková, E. Fialová, D. Bencová and K. Moťková for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vrbová, M., Kotrba, P., Horáček, J. et al. Enhanced accumulation of cadmium in Linum usitatissimum L. plants due to overproduction of metallothionein α-domain as a fusion to β-glucuronidase protein. Plant Cell Tiss Organ Cult 112, 321–330 (2013). https://doi.org/10.1007/s11240-012-0239-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-012-0239-1