Abstract
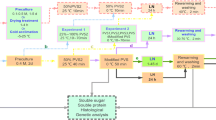
Protocorm-like bodies (PLBs) of Phalaenopsis bellina were successfully cryopreserved by the encapsulation-dehydration approach. Various stages in obtaining successful cryopreservation using this method were optimized. Encapsulated PLBs precultured in half-strength MS medium supplemented with 0.75 M sucrose for 3 days exhibited the highest viability in terms of 2,3,5-triphenyltetrazoliumchloride (TTC) reduction. The amount of sucrose in the PLBs after incubation in different concentrations of sucrose for different periods of time determined by HPLC. The highest sucrose concentration was 7 mg/g of PLBs for the PLBs treated with 0.75 M sucrose for 3 days as compared to the control which had only 1 mg/g sucrose. After sucrose preculture, the PLBs were subjected to desiccation using one of two methods. Desiccation using silica gel was more efficient in reducing PLBs moisture content. After 6 h of desiccation, PLBs desiccated using laminar air flow had 43.5% moisture content while for those desiccated using silica gel had 32% moisture content. PLBs desiccated to different moisture contents were plunged into LN. After storage in LN the encapsulated PLBs were re-warmed. Two weeks after re-warming PLBs viability was determined by TTC reduction and re-growth assessed. Encapsulated PLBs precultured with 0.75 M sucrose for 3 days followed by desiccated using silica gel for 5 h resulting in a moisture content of 39% lead to the highest post re-warming viability in terms of TTC reduction (46.6% of control PLBs) and 30% re-growth.







Similar content being viewed by others
Abbreviations
- MS:
-
Tissue culture medium from Murashige and Skoog (1962)
- NAA:
-
a-Naphthalene acetic acid
- PLBs:
-
Protocorm-like bodies
- LN:
-
Liquid nitrogen
- TTC:
-
Triphenyl tetrazolium chloride
References
Bachiri Y, Bajon C, Sauvanet A, Gazeau C, Morisset C (2000) Effect of osmotic stress on tolerance of air-drying and cryopreservation of Arabidopsis thaliana suspension cells. Protoplasma 214:227–243
Bajaj YPS (1995) Cryopreservation of plant cell, tissue and organ culture for the conservation of germplasm and biodiversity. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry cryopreservation of plant germplasm I. Springer, New York, pp 3–18
Benson EE, Reed BM, Brennan RM, Clacher KA, Ross DA (1996) Use of thermal analysis in the evaluation of cryopreservation protocols for Ribes nigrum L. germplasm. CryoLetters 17:347–362
Bian HW, Wang JH, Lin WQ, Han N, Zhu MY (2002) Accumulation of soluble s u gars, heat-stable proteins and dehydrins in cryopreservation of protocorm-like bodies of Dendrobium candidum by the air-drying method. J Plant Physiol 159:1139–1145
Burritt DJ (2008) Efficient cryopreservation of adventitious shoots of Begonia x erythrophyllausing encapsulation–dehydration requires pretreatment with both ABA and proline. Plant Cell Tissue Organ Cult 95:209–215
Chang MC, Chang SY, Chen SL, Chuang SM (1992) Cloning and expression inEscherichia coli of the gene encoding an extracellular deoxyribonuclease (DNase) from Aeromonas hydrophila. Gene 122:175–180
Chen JT, Chang WC (2004) Induction of repetitive embryogenesis from seed-derived protocorms of Phalaenopsis amabilis var formosa Shimadzu. In Vitro Cell Dev Biol 40:290–293
Datta BK, Kanjilal B, Sarker DD (1999) Artificial seed technology: development of a protocol in Geodorum densiflorum (Lam) Schltr: an endangered orchid. Curr Sci 74:1142–1145
Dumet D, Engelmann F, Chabrillange N, Duval Y, Dereuddre J (1993) Importance of sucrose for the acquisition of tolerance to desiccation and cryopreservation of oil palm somatic embryos. CryoLetters 14:243–250
Duncan DB (1955) Multiple range and multiple F test. Biometrics 11:1–42
Duran-Villa N (1995) Cryopreservation of germplasm of citrus. In: Bajai YPS (ed) Biotechnology in agriculture and forestry: cryopreservation of plant germplasm. Springer, pp 70–86
Engelmann F (1997) In vitro conservation methods. In: Ford-Lloyd BV, Newburry JH, Callow JA (eds) Biotechnology and plant genetic resources: conservation and use. CAB International, Wellingford, pp 119–162
Engelmann F (2000) Importance of cryopreservation for the conservation of plant genetic resources. In: Engelmann F, Takagi H (eds) Cryopreservation of tropical germplasm Current Research Progress and Application. Japan International Research Center for Agricultural Sciences and International Plant Genetic Resources Institute, Rome, pp 8–20
Engelmann F (2009) Cryopreservation for long-term conservation of agrobiodiversity: progress and prospects. A scientific lecture delivered at University Kebangsann Malaysia for the INBIOSIS seminar series
Engelmann F, Takagi H (2000) Cryopreservation of tropical plant germplasm—current research progress and applications. Tsukuba: JIRCAS; Rome: IPGRI
Florin B, Brulard E, Ducos PJ, Tessereau H, Petiard V (2000) Development of a simplified method for the routine cryopreservation of coffee germplasm collection. In: Engelmann F, Hiroko T (eds) Cryopreservation of tropical plant germplasm, Japan/International Plant Genetic Resources Institute, Rome, Italy, pp 496
Gonzalez-Arnoa MT, Panta A, Roca WM, Escobor RH, Engelmann F (2008) Development and large scale application of cryopreservation technique of shoot and somatic embryo cultures of tropical crops. Plant Cell Tissue Organ Cult 92:1–13
Hirano T, Godo T, Mii M, Ishikawa K (2005) Cryopreservation of immature seeds of Bletilla striata by vitrification. Plant Cell Rep 23:534–539
Hirano T, Ishikawa K, Mii M (2006) Advances in orchid crypreservation. Floric Ornam Plant Biotechnol 2:410–414
Hirata K, Goda S, Phunchindawan M, Du D, Ishio M, Sakai A, Miyamoto K (1998) Cryopreservation of horseradish hairy root cultures by encapsulation-dehydration. J Ferment Bioeng 86:418–420
Hitmi A, Barthomeuf C, Sallanon H (1999) Cryopreservation of chrysanthemum cinerariaefolium shoot tips Effects of pretreatment conditions and retention of biosynthetic capacity. CryoLetters 20:109–120
Hoekstra FA, Golovina EA, Buitink J (2001) Mechanisms of plant desiccation tolerance. Trends Plant Sci 6:431–438
Hong SR, Yin MH (2009) High-efficiency vitrification protocols for cryopreservation of in vitro grown shoot tips of rare and endangered plant Emmenopterys henryi Oliv. Plant Cell Tissue Organ Cult 99:217–226
Hsiao YY, Tsai WC, Kuoh CS, Huang TH, Wang HC, Wu TS, Leu YL, Chen WH, Chen HH (2006) Comparison of transcripts in Phalaenopsis bellina and Phalaenopsis equestris (Orchidaceae) flowers to deduce monoterpene biosynthesis pathway. BMC Plant Biol 6:14
Hunt RS, Jackson PA, Mortlok RE, Kirtc RS (1977) Quantitative determination of sugar in foodstuffs by high performance liquid chromatography. Analyst 102:17–20
Ishikawa M, Robertson AJ, Gusta LV (1995) Comparison of viability tes ts for assessing cross -adaptation to freezing, heat and salts tresses induced by abscisic acid in bromegrass (Bromus inermis Leyss) suspension cultured cells. Plant Sci 107:83–93
Ishikawa K, Harata K, Mii M, Sakai A, Yoshimatsu K, Shimomura K (1997) Cryopreservation of a Japanese terrestrial orchid (Bletilla striata) by vitrification. Plant Cell Rep 16:754–757
Islam MO, Rahman ARMM, Matsui S, Prodhan AKMA (2003) Effects of complex organic extracts on callus growth and PLB regeneration through embryogenesis in the Doritaenopsis orchid. Jpn Agric Res Q 37:229–235
ISTA (2005) International rules for seed testing. International Seed Testing Association, Zurich
Jitsopakul N, Thammasiri K, Ishikawa K (2007) Cryopreservation of Vanda coerulea protocorms by encapsulation-dehydration method. In: 33rd Congress on science and technology of Thailand
Khoddamzadeh AA, Sinniah UR, Kadir MA, Kadzimin SB, Maziah M, Sreeramanan S (2010) Detection of Somaclonal variation by random amplified polymorphic DNA analysis during micropropagation of Phalaenopsis bellina (Rchb.f.) christenson. Af J Biotechnol 4:6632–6639
Koster K (1991) Glass formation and desiccation tolerance in seeds. Plant Physiol 96:302–304
Lambardi M, Fabbri A, Caccavale A (2000) Cryopreservation of white poplar (Populus alba L.) by vitrification of in vitro-grown shoot tips. Plant Cell Rep 19:213–218
Langis R, Steponkus PL (1991) Vitrification of isolated rye protoplasts: protection against dehydration injury by ethylene glycol. CryoLetters 12:107–112
Luo JP, Wang Y, Zha XQ, Huang L (2008) Micropropagation of Dendrobium densiflorum Lindl ex Wall. through protocorm-like bodies: effects of plant growth regulators and lanthanoids. Plant Cell Tissue Organ Cult 93:333–340
Lurswijidjarus W, Thammasiri K (2004) Cryopreservation of shoot tips of Dendrobium walter oumae by encapsulation/dehydration. Sci Asia 30:293–299
Mallon R, Barros P, Luzardo A, Gonzalez ML (2007) Encapsulation of moss buds: an efficient method for the in vitro conservation and regeneration of the endangered moss Splachnum ampullaceum. Plant Cell Tissue Organ Cult 88:41–49
Maneerattanarungroj P, Bunnag S, Monthatong M (2007) In vitro conservation of Cleisostoma areitinum (Rchb.f.) garay, rare Thai orchid species by an encapsulation-dehydration method. Asian J Plant Sci 6:1235–1240
Mari A, Amati F, Mingarelli R, Giannotti A, Sebastio G, Colloridi V, Novelli G, Dallapiccola B (1995) Analysis of the elastin gene in 60 patients with clinical diagnosis of Williams syndrome. Hum Genet 96:444–448
Martinez D, Tames SR, Revilla AM (1999) Cryopreservation of in vitro grown shoot-tips of hop (Humulus lupulus L.) using encapsulation/dehydration. Plant Cell Rep 19:59–63
Mikula M, Proell V, Fischer AN, Mikulits W (2006) Activated hepatic stellate cells induce tumor progression of neoplastic hepatocytes in a TGF-beta dependent fashion. J Cell Physiol 209:560–567
Moran M, Cacho M, Fernandez-Tarrago J, Corchete P (1999) A protocol for the cryopreservation of Digitalis thapsi L. cell cultures. CryoLetters 20:193–198
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–479
Muthusamy J, Staines HJ, Benson EE, Mansor M, Krishnapillay B (2005) Optimizing cryo-conservation strategies for scarce Malaysian orthodox rainforest tree germplasm using Taguchi experimental design. Biodiversity Conserv (in press)
Na YH, Kondo K (1996) Cryopreservation of tissue-cultured shoot primordial from shoot apices of cultured protocorms in Vanda pumila following ABA preculture and desiccation. Plant Sci 118:195–201
Niino T, Sakai A (1992) A cryopreservation of alginate-coated in vitrogrown shoot tips of apple, pear and mulberry. Plant Sci 87:199–206
Panis B, Thinh NT (2001) Cryopreservation of Musa germplasm. In: Escalant JV, Sharrock S (eds) INIBAP technical guideline 5. International Network for the Improvement of Banana and Plantain, Montpellier, p 44
Paulet F, Engelmann F, Glaszmann J (1993) Cryopreservation of apices of in vitro plantlets of sugar cane (Saccharum sp hybrids) using encapsulation/dehydration. Plant Cell Rep 12:525–529
Pelah D, Kaushik RA, Nerd A, Mizrahi Y (2003) Validity of in vitro viability tests for predicting respons e of different vine cacti in the field to high and low temperatures. J Prof Assoc Cactus Dev 5:65–71
Pilatti FK, Aguiar T, Simões T, Benson EE, Viana AM (2011) In vitro and cryogenic preservation of plant biodiversity in Brazil. In Vitro Cell Dev Biol—Plant 47:82–98
Plessis P, Leddet C, Dereuddre J (1991) Resistance to dehydration and to freezing in liquid nitrogen of alginate coated shoot tips of grape vine (Vitis vinifera L. cv. Chardonnay) Series-3. Comptes Rendus de 1’Academie-des-Sciences 313:373–380
Popov AS, Popova EV, Nikishina TV, Vysotskaya ON (2006) Cryobank of plant genetic resources in Russian Academy of Sciences. Int J Refrig 29:403–410
Reed BM, Dumet D, DeNoma JS, Benson EE (2001) Validation of cryopreservation protocols for plant germplasm conservation: a pilot study using Ribes L. Biodivers Conserv 10:939–949
Reed BM, Okut NJ, D’Achino Narver L, DeNoma J (2003) Cold storage and cryopreservation of hops (Humulus L.) shoot cultures through application of standard protocols. CryoLetters 24:389–396
Sakai A, Matsumoto T, Hirai D, Niino T (2000) Newly developed encapsulation-dehydration protocol for plant cryopreservation. CryoLetters 21:53–62
Scottez C, Chevreau E, Godard N, Arnaud Y, Duron M, Dereuddre J (1992) Cryopreservation of cold acclimated shoot tips of pear in vitro cultures after encapsulation-dehydration. Cryobiol 29:691–700
Srivastava V, Khan SA, Banerjee S (2009) An evaluation of genetic fidelity of encapsulated microshoots of the medicinal plant:Cineraria maritima following six months of storage. Plant Cell Tissue Organ Cult 99:193–198
Steponkus PL, Lanphear FO (1967) Refinement of the triphenyl tetrazolium chloride method of determining cold injury. Plant Physiol 42:1423–1426
Tokuhara K, Mii M (2003) Highly-efficient somatic embryogenesis from cell suspension cultures of Phalaenopsis orchids by adjusting carbohydrate sources. In Vitro Cell Dev Biol 39:635–639
Touchell DH, Chiang VL, Tsai C-J (2002) Cryopreservation of embryogenic cultures of Picea mariana (black spruce) using vitrification. Plant Cell Rep 21:118–124
Towill LE (1996) Vitrification as a method to cryopreserve shoot tips. In: Trigiano RS, Gray DJ (eds) Plant tissue culture concepts and laboratory exercises. CRC Press, Boca Raton, pp 297–304
Tsai SF, Yeh SD, Chan CF, Liaw SI (2009) High-efficiency vitrification protocols for cryopreservation of in vitro grown shoot tips of transgenic papaya lines. Plant Cell Tissue Organ Cult 98:157–164
Tsukazaki H, Mii M, Tokuhara K, Ishikawa K (2000) Cryopreservation of Doritaenopsis suspension culture by vitrification. Plant Cell Rep 19:1160–1164
Turner SR, Senaratna T, Bunn E, Tan B, Dixon KW, Touchell DH (2001) Cryopreservation of shoot tips from six endangered Austrailian species using a modified vitrification protocol. Annual Bot 87:371–378
Uragami T, Kinoshita H, Okuno H (1993) Characteristics of pereation and separation of aqueous alcholic solutions with chitosan derivative membranes. Die Angewante Makro-molekular Chemie. 209:41
Verleysen H, Samyn G, Van Bockstaele E, Debergh P (2004) Evaluation of analytical techniques to predict viability after cryopreservation. Plant Cell Tissue Organ Cult 77:11–21
Verleysen H, Bockstaele EV, Debergh P (2005) An encapsulation–dehydration protocol for cryopreservation of the azalea cultivar ‘No rd licht’ (Rhododendron simsii Planch.). Sci Hortic 106:402–414
Vertucci CW, Roos EE (1991) Seed moisture content, storage, viability and vigour: response (correspondence). Seed Sci Res 1:277–279
Wang QC, Batuman O, Li P, Bar-Joseph M, Gafny R (2002) Cryopreservation of in vitro-grown shoot tips of ‘Troyer’ citrange [Poncirus trifoliata Raf. 9 Citrus sinensis (L.) Osbeck.] by encapsulation-dehydration. Plant Cell Rep 20:901–906
Wang QC, Mawassi M, Li P, Gafny R, Sela I, Tanne E (2003) Elimination of grapevine virus A (GVA) by cryopreservation of in vitro-grown shoot tips of Vitis vinifera L. Plant Sci 165:321–327
Whiters LA (1985) Cryopres ervation of cultured plant cells and protoplasts. In: Vasil JK (ed) Cell culture and somatic cell genetics of plants. Cell growth, nutrition, cytodifferentiation, and cryopreservation, vol 2. Academic Press Inc., Orlando, pp 253–316
Wusteman M, Pegg D, Robinson M, Wang L, Fitch P (2002) Vitrification media: toxicity, permeability, and dielectric properties 1. Cryobiol 44:24–37
Yin MH, Hong SR (2009) Cryopreservation of Dendrobium candidum wall. ex Lindl. protocorm-like bodies by encapsulation-vitrification. Plant Cell Tissue Organ Cult 98:179–185
Yin MH, Hong SR (2010) A simple cryopreservation protocol of Dioscorea bulbifera L. embryogenic calli by encapsulation-vitrification. Plant Cell Tissue Organ Cult 101:349–358
Young PS, Murthy HN, Yoeup PK (2000) Mass multiplication of protocorm-like bodies using bioreactor system and subsequent plant regeneration in Phalaenopsis. Plant Cell Tissue Org Cult 63:67–72
Zhang YX, Wang JH, Bian H, Zhu MY (2001) Pregrowth desiccation: a simple andefficient procedure for the cryopreservation of rice (Oryza sativa L.) embryogenic suspension cells. CryoLetters 22:221–228
Zhao PF, Wu F, Feng S, Wang WJ (2007) Protocorm-like body (PLB) formation and plan t regeneration from the callus culture of Dendrobium candidum Wall ex Lindl. In Vitro Cell Dev Bio Plant
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khoddamzadeh, A.A., Sinniah, U.R., Lynch, P. et al. Cryopreservation of protocorm-like bodies (PLBs) of Phalaenopsis bellina (Rchb.f.) christenson by encapsulation-dehydration. Plant Cell Tiss Organ Cult 107, 471–481 (2011). https://doi.org/10.1007/s11240-011-9997-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-011-9997-4