Abstract
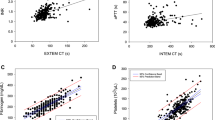
Excessive bleeding after cardiopulmonary bypass (CPB) is risk factor for adverse outcomes after elective cardiac surgery (ECS). Differentiating between patients who bleed due to surgical issues and those whose excessive chest tube output (CTO) is due to coagulopathy, remains challenging. Bedside suitable tests to identify hemostatic disturbances and predict excessive bleeding are desirable. The study sought to evaluate prediction of excessive bleeding after ECS using two bedside suitable devices for platelet function and viscoelastic blood clot properties assessment. We enrolled 148 patients (105 male and 43 female) undergoing ECS in a prospective observational study. Patients were characterized as bleeders if their 24 h CTO exceeded the 75th percentile of distribution. Multiple electrode aggregometry (MEA, with ASPI, ADP and the TRAP test) and rotational thromboelastometry (TEM, with ExTEM, HepTEM and FibTEM test), were performed at three time points: preoperatively (T1), during CPB (T2), and after protamine administration (T3). The primary endpoint was CTO and the secondary endpoint was administration of blood products, 30-day and 1 year mortality. The best predictors of increased bleeding tendency were the tests performed after protamine administration (T3). At T3, patients characterized as bleeders had significantly lower MEA ASPI (median, 14 vs. 27 AUC, p = 0.004) and ADP test values (median, 22 vs. 41 AUC, p = 0.002) as well as TEM values expressed in maximum clot firmness after 30 min (MCF 30) for ExTEM (53 vs. 56 mm, p = 0.005), HepTEM (48 vs. 52 mm, p = 0.003) and FibTEM (8 vs. 11 mm, p < 0.001) test. 24 h CTO inversely correlated with both the MEA (ASPI test: r = −0.236, p = 0.004; ADP test: r = −0.299, p < 0.001), and TEM MCF 30 (ExTEM: r = −0.295, p < 0.001; HepTEM: −0.329, p < 0.001; FibTEM: −0.377, p < 0.001) test values. Our study showed that MEA and TEM are useful methods for prediction of excessive bleeding after ECS. In order to prevent excessive postoperative CTO, hemostatic interventions with timely and targeted blood component therapy according to MEA and TEM results should be considered.





Similar content being viewed by others
References
Fergusson DA, Hebert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, Teoh K, Duke PC, Arellano R, Blajchman MA, Bussieres JS, Cote D, Karski J, Martineau R, Robblee JA, Rodger M, Wells G, Clinch J, Pretorius R (2008) A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med 358:2319–2331
Woodman RC, Harker LA (1990) Bleeding complications associated with cardiopulmonary bypass. Blood 76:1680–1697
Dixon B, Santamaria JD, Reid D, Collins M, Rechnitzer T, Newcomb AE, Nixon I, Yii M, Rosalion A, Campbell DJ (2012) The association of blood transfusion with mortality after cardiac surgery: cause or confounding? Transfusion. doi:10.1111/j.1537-2995.2012.03697.x
Practice Guidelines for blood component therapy: a report by the American Society of Anesthesiologists Task Force on Blood Component Therapy (1996) Anesthesiology 84:732–747
Lackritz EM, Satten GA, Aberle-Grasse J, Dodd RY, Raimondi VP, Janssen RS, Lewis WF, Notari EP, Petersen LR (1995) Estimated risk of transmission of the human immunodeficiency virus by screened blood in the United States. N Engl J Med 333:1721–1725
Tremolada F, Chiappetta F, Noventa F, Valfre C, Ongaro G, Realdi G (1983) Prospective study of posttransfusion hepatitis in cardiac surgery patients receiving only blood or also blood products. Vox Sang 44:25–30
Fries D, Streif W, Haas T, Kuhbacher G (2004) Dilutional coagulopathy, an underestimated problem? Anasthesiol Intensivmed Notfallmed Schmerzther 39:745–750
Edmunds LH Jr, Ellison N, Colman RW, Niewiarowski S, Rao AK, Addonizio VP Jr, Stephenson LW, Edie RN (1982) Platelet function during cardiac operation: comparison of membrane and bubble oxygenators. J Thorac Cardiovasc Surg 83:805–812
Harker LA, Malpass TW, Branson HE, Hessel EA 2nd, Slichter SJ (1980) Mechanism of abnormal bleeding in patients undergoing cardiopulmonary bypass: acquired transient platelet dysfunction associated with selective alpha-granule release. Blood 56:824–834
Mammen EF, Koets MH, Washington BC, Wolk LW, Brown JM, Burdick M, Selik NR, Wilson RF (1985) Hemostasis changes during cardiopulmonary bypass surgery. Semin Thromb Hemost 11:281–292
Gundry SR, Drongowski RA, Klein MD, Coran AG (1989) Postoperative bleeding in cardiovascular surgery. Does heparin rebound really exist? Am Surg 55:162–165
Tanaka K, Takao M, Yada I, Yuasa H, Kusagawa M, Deguchi K (1989) Alterations in coagulation and fibrinolysis associated with cardiopulmonary bypass during open heart surgery. J Cardiothorac Anesth 3:181–188
Rinder CS, Bohnert J, Rinder HM, Mitchell J, Ault K, Hillman R (1991) Platelet activation and aggregation during cardiopulmonary bypass. Anesthesiology 75:388–393
Mengistu AM, Wolf MW, Boldt J, Rohm KD, Lang J, Piper SN (2008) Evaluation of a new platelet function analyzer in cardiac surgery: a comparison of modified thromboelastography and whole-blood aggregometry. J Cardiothorac Vasc Anesth 22:40–46
Spiess BD, Tuman KJ, McCarthy RJ, DeLaria GA, Schillo R, Ivankovich AD (1987) Thromboelastography as an indicator of post-cardiopulmonary bypass coagulopathies. J Clin Monit 3:25–30
Ti LK, Cheong KF, Chen FG (2002) Prediction of excessive bleeding after coronary artery bypass graft surgery: the influence of timing and heparinase on thromboelastography. J Cardiothorac Vasc Anesth 16:545–550
Goodnough LT, Soegiarso RW, Birkmeyer JD, Welch HG (1993) Economic impact of inappropriate blood transfusions in coronary artery bypass graft surgery. Am J Med 94:509–514
Nightingale CH, Robotti J, Deckers PJ, Allmendinger PD, Lowe R, Low HB (1987) Quality care and cost-effectiveness. An organized approach to problem solving. Arch Surg 122:451–456
Spiess BD, Gillies BS, Chandler W, Verrier E (1995) Changes in transfusion therapy and reexploration rate after institution of a blood management program in cardiac surgical patients. J Cardiothorac Vasc Anesth 9:168–173
Toth O, Calatzis A, Penz S, Losonczy H, Siess W (2006) Multiple electrode aggregometry: a new device to measure platelet aggregation in whole blood. Thromb Haemost 96:781–788
Calatzis A, Krueger WB (2004) A new approach to platelet function analysis in whole blood- the multiplate analyzer. Platelets 15:479–517
Cammerer U, Dietrich W, Rampf T, Braun SL, Richter JA (2003) The predictive value of modified computerized thromboelastography and platelet function analysis for postoperative blood loss in routine cardiac surgery. Anesth Analg 96:51–57, table of contents
Metz CE (1978) Basic principles of ROC analysis. Semin Nucl Med 8:283–298
Paparella D, Brister SJ, Buchanan MR (2004) Coagulation disorders of cardiopulmonary bypass: a review. Intensive Care Med 30:1873–1881
Harker LA (1987) Acquired disorders of platelet function. Ann NY Acad Sci 509:188–204
Essell JH, Martin TJ, Salinas J, Thompson JM, Smith VC (1993) Comparison of thromboelastography to bleeding time and standard coagulation tests in patients after cardiopulmonary bypass. J Cardiothorac Vasc Anesth 7:410–415
Forestier F, Coiffic A, Mouton C, Ekouevi D, Chene G, Janvier G (2002) Platelet function point-of-care tests in post-bypass cardiac surgery: are they relevant? Br J Anaesth 89:715–721
Ray MJ, Marsh NA, Hawson GA (1994) Relationship of fibrinolysis and platelet function to bleeding after cardiopulmonary bypass. Blood Coagul Fibrinolysis 5:679–685
Nuttall GA, Oliver WC, Ereth MH, Santrach PJ (1997) Coagulation tests predict bleeding after cardiopulmonary bypass. J Cardiothorac Vasc Anesth 11:815–823
Dorman BH, Spinale FG, Bailey MK, Kratz JM, Roy RC (1993) Identification of patients at risk for excessive blood loss during coronary artery bypass surgery: thromboelastography versus coagulation screen. Anesth Analg 76:694–700
Wang JS, Lin CY, Hung WT, O’Connor MF, Thisted RA, Lee BK, Karp RB, Yang MW (1992) Thromboelastogram fails to predict postoperative hemorrhage in cardiac patients. Ann Thorac Surg 53:435–439
Ostrowsky J, Foes J, Warchol M, Tsarovsky G, Blay J (2004) Plateletworks platelet function test compared to the thromboelastograph for prediction of postoperative outcomes. J Extra Corpor Technol 36:149–152
Royston D, von Kier S (2001) Reduced haemostatic factor transfusion using heparinase-modified thrombelastography during cardiopulmonary bypass. Br J Anaesth 86:575–578
Reinhofer M, Brauer M, Franke U, Barz D, Marx G, Losche W (2008) The value of rotation thromboelastometry to monitor disturbed perioperative haemostasis and bleeding risk in patients with cardiopulmonary bypass. Blood Coagul Fibrinolysis 19:212–219
Nielsen VG (2007) A comparison of the thrombelastograph and the TEM. Blood Coagul Fibrinolysis 18:247–252
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petricevic, M., Biocina, B., Milicic, D. et al. Bleeding risk assessment using whole blood impedance aggregometry and rotational thromboelastometry in patients following cardiac surgery. J Thromb Thrombolysis 36, 514–526 (2013). https://doi.org/10.1007/s11239-013-0868-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-013-0868-1